
A closed gas cylinder is divided into two parts by a piston held by a piston held tight. The pressure and the volume of gas in two parts respectively are (p, 5V) and (10p, V). If now the piston is left free and the system undergoes isothermal process, then the volume of the gas in two parts respectively are:
A.) 4V, 2V
B.) 5V, V
C.) 2V, 4V
D.) 3V, 3V
Answer
605.7k+ views
Hint: An important point to be noted is that in the isothermal process, the temperature remains constant. So, the ideal gas equation, i.e., $PV = nRT$ , so if T is constant due to an isothermal process that means PV remains constant, use this concept to solve further.
Complete step-by-step answer:
Formula used - $PV = nRT$ , ${P_1}{V_1} = {P_2}{V_2}$
We have been given a closed cylinder divided into two parts held by a piston.
Also, the pressure and the volume of the gas in the two parts are (p, 5V) and (10p, V).
For better understanding refer the figure below-
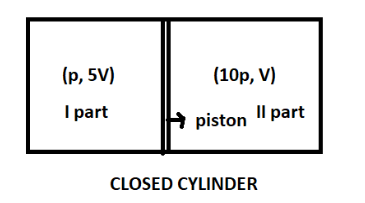
Now, the above figure shows a closed cylinder divided into two parts held by a piston and the pressure and volume of the gas in the two parts.
Now, as given in the question, if the piston is left free the system undergoes an isothermal process.
So, as we know that in an isothermal process the temperature remains constant.
So, using the ideal gas equation-
$PV = nRT$
So, as in isothermal process the temperature is constant, so in the above equation T is constant and n i.e. the number of moles and R is the universal gas constant.
So, the right-hand side of the equation is constant, which implies PV = constant.
Let ${P_1},{V_1}$ are the pressure and volume of the gas in part I and ${P_2},{V_2}$ are the pressure and volume of the gas in part II.
So, as already given in the question that the pressure and volume of the gas in part I and II are- (p, 5V) and (10p, V).
Now, as the piston is left free the piston will move towards the lower pressure side till the pressure becomes equal on both sides.
So, let the common pressure be p’.
So, we can now apply PV = constant and we can write-
${P_1}{V_1} + {P_2}{V_2} = p'V - (1)$
Here ${P_1} = p,{V_1} = 5V,{P_2} = 10p,{V_2} = V$ and p’ is the common pressure and V is the total volume which will be- $V = {V_1} + {V_2} = 5V + V = 6V$
So, keeping the values in equation (1), we get-
$
p \times 5V + 10p \times V = p' \times 6V \\
\Rightarrow 5pV + 10pV = 6p'V \\
\Rightarrow 15pV = 6p'V \\
\Rightarrow p' = \dfrac{{15}}{6}p = \dfrac{5}{2}p \\
$
Now, for I part if PV = constant (Boyle’s Law) so-
$p \times 5V = p' \times {V_1}'$
Here, $V{'_1}$ is the volume in the first part after undergoing an isothermal process.
Putting the values; we get-
$
p \times 5V = \dfrac{5}{2}p \times {V_1}' \\
\Rightarrow 5pV = \dfrac{5}{2}P{V_1}' \\
\Rightarrow {V_1}' = 2V \\
$
Similarly, using Boyle’s Law, PV = constant for part II-
$10p \times V = p' \times {V_2}'$
Here, ${V_2}'$ is the volume in the second part after undergoing an isothermal process.
Putting the values; we get-
$
10p \times V = \dfrac{5}{2}p \times {V_2}' \\
\Rightarrow 10pV = \dfrac{5}{2}P{V_2}' \\
\Rightarrow {V_2}' = 4V \\
$
Therefore, the volume of the part I and part II are 2V and 4V respectively.
Hence, the correct option is C.
Note: Whenever such types of questions appear, first write down the information given in the question and then draw a figure for understanding the question properly, as mentioned in the question. As, isothermal process occurs, so PV will remain constant and also if the piston is allowed to move freely so it will move towards the lower pressure region, which is part I, till the pressure becomes same for both the parts, then by applying Boyle’s law i.e., PV = constant we have find the volume in the both the parts.
Complete step-by-step answer:
Formula used - $PV = nRT$ , ${P_1}{V_1} = {P_2}{V_2}$
We have been given a closed cylinder divided into two parts held by a piston.
Also, the pressure and the volume of the gas in the two parts are (p, 5V) and (10p, V).
For better understanding refer the figure below-

Now, the above figure shows a closed cylinder divided into two parts held by a piston and the pressure and volume of the gas in the two parts.
Now, as given in the question, if the piston is left free the system undergoes an isothermal process.
So, as we know that in an isothermal process the temperature remains constant.
So, using the ideal gas equation-
$PV = nRT$
So, as in isothermal process the temperature is constant, so in the above equation T is constant and n i.e. the number of moles and R is the universal gas constant.
So, the right-hand side of the equation is constant, which implies PV = constant.
Let ${P_1},{V_1}$ are the pressure and volume of the gas in part I and ${P_2},{V_2}$ are the pressure and volume of the gas in part II.
So, as already given in the question that the pressure and volume of the gas in part I and II are- (p, 5V) and (10p, V).
Now, as the piston is left free the piston will move towards the lower pressure side till the pressure becomes equal on both sides.
So, let the common pressure be p’.
So, we can now apply PV = constant and we can write-
${P_1}{V_1} + {P_2}{V_2} = p'V - (1)$
Here ${P_1} = p,{V_1} = 5V,{P_2} = 10p,{V_2} = V$ and p’ is the common pressure and V is the total volume which will be- $V = {V_1} + {V_2} = 5V + V = 6V$
So, keeping the values in equation (1), we get-
$
p \times 5V + 10p \times V = p' \times 6V \\
\Rightarrow 5pV + 10pV = 6p'V \\
\Rightarrow 15pV = 6p'V \\
\Rightarrow p' = \dfrac{{15}}{6}p = \dfrac{5}{2}p \\
$
Now, for I part if PV = constant (Boyle’s Law) so-
$p \times 5V = p' \times {V_1}'$
Here, $V{'_1}$ is the volume in the first part after undergoing an isothermal process.
Putting the values; we get-
$
p \times 5V = \dfrac{5}{2}p \times {V_1}' \\
\Rightarrow 5pV = \dfrac{5}{2}P{V_1}' \\
\Rightarrow {V_1}' = 2V \\
$
Similarly, using Boyle’s Law, PV = constant for part II-
$10p \times V = p' \times {V_2}'$
Here, ${V_2}'$ is the volume in the second part after undergoing an isothermal process.
Putting the values; we get-
$
10p \times V = \dfrac{5}{2}p \times {V_2}' \\
\Rightarrow 10pV = \dfrac{5}{2}P{V_2}' \\
\Rightarrow {V_2}' = 4V \\
$
Therefore, the volume of the part I and part II are 2V and 4V respectively.
Hence, the correct option is C.
Note: Whenever such types of questions appear, first write down the information given in the question and then draw a figure for understanding the question properly, as mentioned in the question. As, isothermal process occurs, so PV will remain constant and also if the piston is allowed to move freely so it will move towards the lower pressure region, which is part I, till the pressure becomes same for both the parts, then by applying Boyle’s law i.e., PV = constant we have find the volume in the both the parts.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




