
A charged oil drop of mass $2.5 \times {10^{ - 7}}kg$ is in space between two plates, each of area $2 \times {10^{ - 2}}{m^2}$ of a parallel plate capacitor. When the upper plate has a charge of $5 \times {10^{ - 7}}C$ and the lower plate has equal negative charge then the oil remains stationary. The charge on the oil drop is $(\text take, \text g = 10m/{s^2})$
a. $9 \times {10^{ - 3}}C$
b. $9 \times {10^{ - 6}}C$
c. $8.85 \times {10^{ - 13}}C$
d. $1.8 \times {10^{ - 34}}C$
Answer
595.5k+ views
Hint: The oil drop is stationary between the plates which means the net force on the oil drop is zero. The upward electrostatic force is balanced by downward force of gravity.
Step by step answer:
Following information is given in the question
Mass of oil drop, $m = 2.5 \times {10^{ - 7}}kg$
Area of parallel plates, $A = 2 \times {10^{ - 2}}{m^2}$
Charge on oil drop,$Q = 5 \times {10^{ - 7}}C$
Now,
We know that Surface charge density,
\[\lambda = \dfrac{Q}{a}\]
$\lambda = \dfrac{{5 \times {{10}^{ - 7}}C}}{{2 \times {{10}^{ - 2}}{m^2}}}$
$\lambda = 2.5 \times {10^{ - 5}}C/{m^2}$
We know that,
Electric field inside a parallel plate capacitor,$E = \dfrac{\lambda }{{{\varepsilon _0}}}$
Where,
${\varepsilon _0} = $permittivity of free space
$E = \dfrac{{2.5 \times {{10}^{ - 5}}}}{{8.85 \times {{10}^{ - 12}}}}$
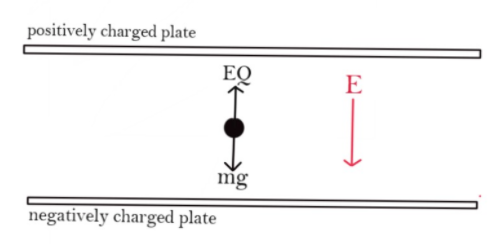
Balancing forces on oil drop
Gravitational force = electrostatic force
$mg = EQ$
$2.5 \times {10^{ - 7}} \times 10 = \dfrac{{2.5 \times {{10}^{ - 5}}}}{{8.85 \times {{10}^{ - 12}}}} \times Q$
$Q = 8.85 \times {10^{ - 13}}C$
Option C is the correct answer
Electric field given in the question is downwards as shown in the figure. So charge must be negatively charged in order to balance the force of gravity by applying electrostatic force in an upward direction.
$Q = - 8.85 \times {10^{ - 13}}C$
Additional information:
- This is a famous experiment known as the Millikan’s oil drop experiment. This experiment is performed by Robert A. Millikan to measure the electric charge of an electron.
- The goal of this experiment was to observe tiny charged droplets of oil between two horizontal electrodes. The only difference between this question and Millikan’s experiment is that the drops are not in rest in the original experiment. The droplets are allowed to fall freely between the electrode and terminal velocity is noted which is then used to calculate the electric charge of an electron.
- Millikan repeated this experiment number of times then he obtained various values of charge and is found to be a multiple of $1.6 \times {10^{ - 19}}C$
Note: Electric field produced by single plate is $\dfrac{\lambda }{{2{\varepsilon _0}}}$ but electric field inside the parallel plate capacitor is the sum of electric fields produced by both plates which is $2 \times \dfrac{\lambda }{{2{\varepsilon _0}}} = \dfrac{\lambda }{{{\varepsilon _0}}}$.
Step by step answer:
Following information is given in the question
Mass of oil drop, $m = 2.5 \times {10^{ - 7}}kg$
Area of parallel plates, $A = 2 \times {10^{ - 2}}{m^2}$
Charge on oil drop,$Q = 5 \times {10^{ - 7}}C$
Now,
We know that Surface charge density,
\[\lambda = \dfrac{Q}{a}\]
$\lambda = \dfrac{{5 \times {{10}^{ - 7}}C}}{{2 \times {{10}^{ - 2}}{m^2}}}$
$\lambda = 2.5 \times {10^{ - 5}}C/{m^2}$
We know that,
Electric field inside a parallel plate capacitor,$E = \dfrac{\lambda }{{{\varepsilon _0}}}$
Where,
${\varepsilon _0} = $permittivity of free space
$E = \dfrac{{2.5 \times {{10}^{ - 5}}}}{{8.85 \times {{10}^{ - 12}}}}$

Balancing forces on oil drop
Gravitational force = electrostatic force
$mg = EQ$
$2.5 \times {10^{ - 7}} \times 10 = \dfrac{{2.5 \times {{10}^{ - 5}}}}{{8.85 \times {{10}^{ - 12}}}} \times Q$
$Q = 8.85 \times {10^{ - 13}}C$
Option C is the correct answer
Electric field given in the question is downwards as shown in the figure. So charge must be negatively charged in order to balance the force of gravity by applying electrostatic force in an upward direction.
$Q = - 8.85 \times {10^{ - 13}}C$
Additional information:
- This is a famous experiment known as the Millikan’s oil drop experiment. This experiment is performed by Robert A. Millikan to measure the electric charge of an electron.
- The goal of this experiment was to observe tiny charged droplets of oil between two horizontal electrodes. The only difference between this question and Millikan’s experiment is that the drops are not in rest in the original experiment. The droplets are allowed to fall freely between the electrode and terminal velocity is noted which is then used to calculate the electric charge of an electron.
- Millikan repeated this experiment number of times then he obtained various values of charge and is found to be a multiple of $1.6 \times {10^{ - 19}}C$
Note: Electric field produced by single plate is $\dfrac{\lambda }{{2{\varepsilon _0}}}$ but electric field inside the parallel plate capacitor is the sum of electric fields produced by both plates which is $2 \times \dfrac{\lambda }{{2{\varepsilon _0}}} = \dfrac{\lambda }{{{\varepsilon _0}}}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




