
A bond cleavage can occur by two ways, homolytic and heterolytic fission. What happens to ${{CH}}$ bond when it undergoes a heterolytic fission?
Answer
573.6k+ views
Hint:Breaking of a bond is called bond cleavage. A bond can break in two ways, homolytically and heterolytically meaning that the electrons in a covalent bond are shared between two atoms unequally or the electrons are shared between the atoms equally.
Complete step by step solution:
Organic compounds are usually formed by covalent bonds, which involves sharing of electrons between two atoms. The atoms forming the covalent bond break apart from each other in two ways:
Homolytic fission
Heterolytic fission
Homolytic fission
Homolytic fission is a type of bond fission in which the two electrons used for forming a bond or the bonding pair of electrons is equally divided between the bonding atoms when the bond breaks. Therefore, the homolytic fission of a neutral molecule results in the formation of two free radicals as the products. Homolytic fission is also known as homolytic cleavage or bond homolysis. This type of bond cleavage happens under certain specific conditions like ultraviolet rays, high temperatures or high temperatures in the absence of oxygen to facilitate pyrolysis. The energy required for homolytic fission in a molecule is called homolytic bond dissociation energy.
Homolytic fission is depicted by drawing two fish-hook arrows across the bond, pointing towards the two atoms involved in the bond formation.
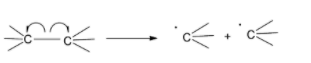
Heterolytic bond fission
Heterolytic bond fission is a type of bond fission in which the two electrons involved in forming a bond or the bonding pair of electrons is divided unequally between the bonding atoms when the bond breaks. Therefore, the heterolytic fission of a neutral molecule results in the formation of one negatively charged atom and another positively charged atom. Heterolytic fission is also known as heterolytic cleavage or bond heterolysis. The main driving force for this type of cleavage to happen is the difference in electronegativities of the bonding atoms. The atom with higher electronegativity tends to retain the bonding pair of electrons with itself thereby acquiring a negative charge on it, while the atom with lower electronegativity tends to acquire a positive charge on it. The energy required for the heterolytic fission in a molecule is called heterolytic bond dissociation energy.
Heterolytic fission is depicted by drawing a curly or curved arrow across the bond pointing towards the most electronegative atom involved in the bond formation.
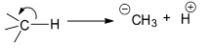
Heterolytic fission of a ${{CH}}$ bond leads to the unequal distribution of the bonding pair of electrons with ${{C}}$ and ${{H}}$ atoms. The deciding factor here, is the electronegativity of the ${{C}}$ and ${{H}}$ atoms. According to Pauling, the electronegativity of ${{C}}$ is $2.55$ and ${{H}}$ is $2.20$ . Hence, the bonding pair of electrons will be shifted to ${{C}}$ carrying a higher electronegativity value.
Note:
Heterolytic fission normally happens in cases where there is a large difference in the electronegativities of the atoms involved in bond formation. Homolytic fission usually happens in cases where the difference in electronegativities of the atoms involved in bond formation is not significantly high.
Complete step by step solution:
Organic compounds are usually formed by covalent bonds, which involves sharing of electrons between two atoms. The atoms forming the covalent bond break apart from each other in two ways:
Homolytic fission
Heterolytic fission
Homolytic fission
Homolytic fission is a type of bond fission in which the two electrons used for forming a bond or the bonding pair of electrons is equally divided between the bonding atoms when the bond breaks. Therefore, the homolytic fission of a neutral molecule results in the formation of two free radicals as the products. Homolytic fission is also known as homolytic cleavage or bond homolysis. This type of bond cleavage happens under certain specific conditions like ultraviolet rays, high temperatures or high temperatures in the absence of oxygen to facilitate pyrolysis. The energy required for homolytic fission in a molecule is called homolytic bond dissociation energy.
Homolytic fission is depicted by drawing two fish-hook arrows across the bond, pointing towards the two atoms involved in the bond formation.

Heterolytic bond fission
Heterolytic bond fission is a type of bond fission in which the two electrons involved in forming a bond or the bonding pair of electrons is divided unequally between the bonding atoms when the bond breaks. Therefore, the heterolytic fission of a neutral molecule results in the formation of one negatively charged atom and another positively charged atom. Heterolytic fission is also known as heterolytic cleavage or bond heterolysis. The main driving force for this type of cleavage to happen is the difference in electronegativities of the bonding atoms. The atom with higher electronegativity tends to retain the bonding pair of electrons with itself thereby acquiring a negative charge on it, while the atom with lower electronegativity tends to acquire a positive charge on it. The energy required for the heterolytic fission in a molecule is called heterolytic bond dissociation energy.
Heterolytic fission is depicted by drawing a curly or curved arrow across the bond pointing towards the most electronegative atom involved in the bond formation.

Heterolytic fission of a ${{CH}}$ bond leads to the unequal distribution of the bonding pair of electrons with ${{C}}$ and ${{H}}$ atoms. The deciding factor here, is the electronegativity of the ${{C}}$ and ${{H}}$ atoms. According to Pauling, the electronegativity of ${{C}}$ is $2.55$ and ${{H}}$ is $2.20$ . Hence, the bonding pair of electrons will be shifted to ${{C}}$ carrying a higher electronegativity value.
Note:
Heterolytic fission normally happens in cases where there is a large difference in the electronegativities of the atoms involved in bond formation. Homolytic fission usually happens in cases where the difference in electronegativities of the atoms involved in bond formation is not significantly high.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




