
(a) Account for the following :
(i) $C{H_3}CHO$ is more reactive than $C{H_3}COC{H_3}$ towards reaction with $HCN$ .
(ii) Carboxylic acid is a stronger acid than phenol.
(b) Write the chemical equations to illustrate the following name reactions:
(i) Wolff-Kishner reduction
(ii) Aldol condensation
(iii) Cannizzaro reaction
Answer
595.2k+ views
Hint: We can account for the reason of $C{H_3}CHO$ is more reactive than $C{H_3}COC{H_3}$ towards reaction with $HCN$ with the help of side groups attached to the carbonyl carbon atoms and in the (iI) part can be solved with the help of stability of the conjugate base of the given acids.
Complete step by step answer:
(a)
(i) $C{H_3}CHO$ is more reactive than $C{H_3}COC{H_3}$ towards reaction with $HCN$ - In the given compound $C{H_3}CHO$ the magnitude of the positive charge on the carbonyl carbon atom reduces because of the presence of methyl group $\left( {C{H_3} - } \right)$. As methyl groups reduce the positive charge because of its $ + I$ effect. In $C{H_3}COC{H_3}$ there are two methyl groups attached to the carbonyl group which also hinders the approach of $C{N^ - }$. Therefore $C{H_3}CHO$ is more reactive than $C{H_3}COC{H_3}$ towards reaction with $HCN$.
(ii) Carboxylic acid is a stronger acid than phenol – We know that the acid will be more acidic if its conjugate base will be more stable. The conjugate base of carboxylic acid and phenol are carboxylate ion and phenoxide ion respectively. The resonance structure of carboxylate ion is more stable than phenoxide ion because the negative charge is delocalised into the benzene ring to a smaller extent while in carboxylate ion it is delocalised on oxygen atom.
(b)
(i) Wolff-Kishner reduction – The Wolff Kishner reduction is performed with ketones or aldehyde substrate and hydrazine as a reducing agent in the presence of a strong base in a high boiling protic solvent such as ethylene glycerol. The chemical equation of the given reaction is as follows;
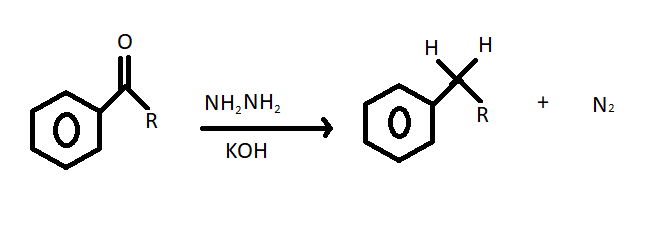
(ii) Aldol condensation - We have studied that $\beta - $ hydroxy aldehydes are known as aldols. Aldol condensation reaction occurs with aldehyde having $\alpha - $hydrogen with a dilute base to give aldols. The simple chemical equation of aldol condensation is given below;
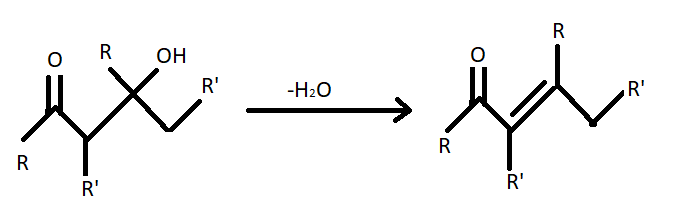
(iii) Cannizzaro reaction – Cannizzaro reaction is redox disproportionation of non-enolizable aldehydes to carboxylic acids and alcohols. It is conducted in a concentrated base. It produces a primary alcohol and a carboxylic acid.
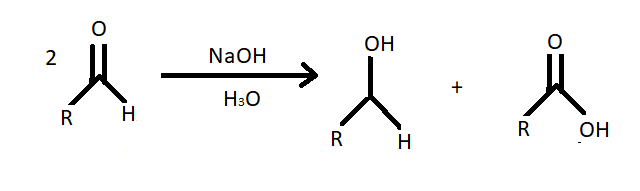
Note:
1- Hence the above problems are solved with the help of some important concepts of chemistry such as the induction effect of methyl group and resonating structure of conjugate bases.
2- These are very important reactions of aldehydes and ketones. They are mainly famous by their names such as Wolff-Kishner reduction, Cannizzaro reaction etc.
Complete step by step answer:
(a)
(i) $C{H_3}CHO$ is more reactive than $C{H_3}COC{H_3}$ towards reaction with $HCN$ - In the given compound $C{H_3}CHO$ the magnitude of the positive charge on the carbonyl carbon atom reduces because of the presence of methyl group $\left( {C{H_3} - } \right)$. As methyl groups reduce the positive charge because of its $ + I$ effect. In $C{H_3}COC{H_3}$ there are two methyl groups attached to the carbonyl group which also hinders the approach of $C{N^ - }$. Therefore $C{H_3}CHO$ is more reactive than $C{H_3}COC{H_3}$ towards reaction with $HCN$.
(ii) Carboxylic acid is a stronger acid than phenol – We know that the acid will be more acidic if its conjugate base will be more stable. The conjugate base of carboxylic acid and phenol are carboxylate ion and phenoxide ion respectively. The resonance structure of carboxylate ion is more stable than phenoxide ion because the negative charge is delocalised into the benzene ring to a smaller extent while in carboxylate ion it is delocalised on oxygen atom.
(b)
(i) Wolff-Kishner reduction – The Wolff Kishner reduction is performed with ketones or aldehyde substrate and hydrazine as a reducing agent in the presence of a strong base in a high boiling protic solvent such as ethylene glycerol. The chemical equation of the given reaction is as follows;

(ii) Aldol condensation - We have studied that $\beta - $ hydroxy aldehydes are known as aldols. Aldol condensation reaction occurs with aldehyde having $\alpha - $hydrogen with a dilute base to give aldols. The simple chemical equation of aldol condensation is given below;

(iii) Cannizzaro reaction – Cannizzaro reaction is redox disproportionation of non-enolizable aldehydes to carboxylic acids and alcohols. It is conducted in a concentrated base. It produces a primary alcohol and a carboxylic acid.

Note:
1- Hence the above problems are solved with the help of some important concepts of chemistry such as the induction effect of methyl group and resonating structure of conjugate bases.
2- These are very important reactions of aldehydes and ketones. They are mainly famous by their names such as Wolff-Kishner reduction, Cannizzaro reaction etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




