




Why Does Water Behave Differently? Key Properties Explained
Water is necessary for the survival of all living things, from microscopic bacteria to enormous blue whales. Life as we know it would not exist without water, and where there is water, there is life.

Properties of Water for Kids
All living things, including plants and animals, depend on water, whether hot or cold, salty or fresh, abundant or scarce. They can survive in various environments, from hot deserts to the chilly, deep oceans. Nearly four billion years ago, the first living organisms emerged in the ocean. Some organisms, those which came before us, adapted to life on land. In addition to deserts and swamps, humans have mastered a variety of other settings.
There are still more different species of life on Earth in the water than any place else.
Properties of Water for Kids:
Properties of Water
Following are the properties of water for kids-
Water Has Two Poles.
The structure of water molecules is twisted and polar, with partial positive charges on the hydrogens and partial negative charges on the oxygen.
A Great Solvent is Water.
Many polar and ionic chemicals can be dissolved by water, which is a unique property. All living creatures need to know this since the water cycle removes many critical nutrients from the water as it moves through it.
Water Vaporizes at a High Temperature.
Humans and other sweating animals use the high heat of vaporization of water to cool themselves. When the temperature for vaporization is attained, water changes from its liquid state to steam.
Cohesive and Adhesive Qualities are Present in Water.
Because water molecules can create hydrogen bonds with one another, they have powerful adhesive forces. Water may adhere to objects other than itself thanks to its adhesive qualities as well.
As a Solid, Water is Less Dense Than as a Liquid.
When water freezes, the molecules organize into a crystalline structure that spreads them farther apart than when the water is liquid. As a result, ice floats because it is less thick than liquid water.
Uses of Water for Kids:
Uses of Water
Both direct and indirect uses of water are possible. While bathing, drinking, and cooking are examples of direct uses of water, indirect uses include the production of steel for automobiles and the processing of wood to manufacture paper. Water is mostly used for agriculture, manufacturing, and energy around the world. The most typical uses of water for kids include-
Drinking and Household Needs
Recreation
Industry and Commerce
Agriculture
Thermoelectricity/Energy
States of Water for Kids:

States of Water
We are aware that water is not always "wet," as it can occasionally be frozen into ice or snow. Water can exist in three different states depending on the temperature and kinetic energy of its molecules.
Following are the states of water for kids-
The solid form of water is called ice. Water freezes and turns into ice when the temperature falls below 0 degrees C (32 degrees F).
The wet substance we consume and swim in is referred to as liquid.
Water converts into its gaseous state, known as vapor, when it evaporates or reaches a temperature of more than 100 degrees Celsius and begins to boil.
Water Formula in Science:
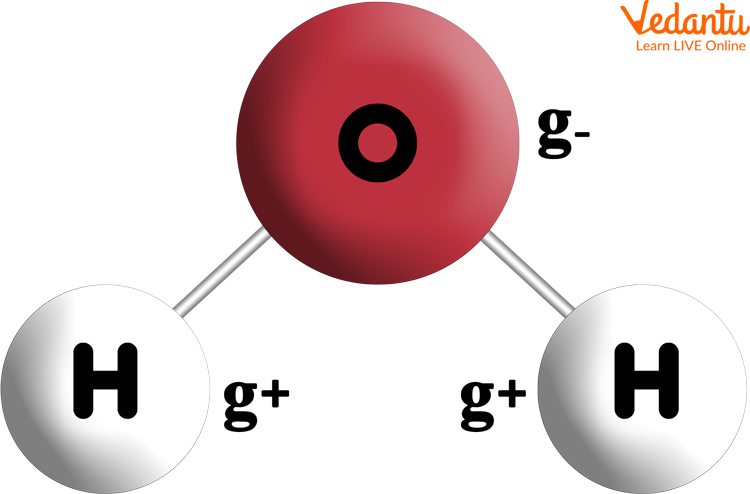
Water Formula in Science
Water formula in science is H2O. One oxygen atom is covalently joined to two hydrogen atoms to form a single water molecule.
Contrary to what most people believe, only totally pure water is empty of all other ions and elements. Chlorine, silicates, magnesium, calcium, aluminum, sodium, and tiny amounts of other ions and compounds are typically found in drinking water.
Additionally, water decomposes into its own H+ and OH- ions.
Summary
Water H2O is an inorganic chemical that exists in liquid, gaseous (steam, water vapor), and solid (ice) phases. Water is a colorless, flavorless, and odorless liquid at normal temperature. Water is one of the most prevalent substances and makes up around 75% of the surface of the planet.
FAQs on Properties of Water for Kids: Hands-On Science Exploration
1. What are the three main forms or states of water found on Earth?
Water exists in three primary states, and we can see examples of each in our daily lives. These states are:
- Solid: This is water in its frozen form, such as ice cubes, snow on mountains, and frost on a cold morning.
- Liquid: This is the most common form we see and use. It is the water in our rivers, lakes, oceans, and the water we drink.
- Gas: This is an invisible gas called water vapour. It is present in the air all around us. When water boils, it turns into steam, which is a visible form of this gas.
2. What are the basic properties of pure water that a child can observe?
For kids learning about water, the most important observable properties are its physical characteristics. Pure water is a unique substance because it is naturally colourless (it has no colour), odourless (it has no smell), and tasteless. It is also transparent, which means you can see through it clearly.
3. Why is water often called the 'universal solvent'?
Water is called the universal solvent because it has the amazing ability to dissolve more substances than any other liquid. When you stir salt or sugar into water, they seem to disappear because their particles have been broken down and spread throughout the water. This property is very important for life, as it helps carry essential nutrients in the soil to plants and within our own bodies.
4. How does water help plants and animals to live?
Water is essential for all living things and plays many important roles. For example:
- It makes up a large part of the body of every plant and animal.
- In animals, it helps digest food and transport nutrients to cells.
- It helps regulate body temperature; for instance, humans sweat to cool down.
- Plants need water to absorb nutrients from the soil and for the process of photosynthesis, which is how they make their own food.
5. Why does ice float on water instead of sinking?
This is one of water's most special properties. When water freezes and turns into ice, its molecules spread out, making the ice less dense than the liquid water around it. Because it is lighter for its size, ice floats. This is crucial for aquatic life in cold climates, as the floating layer of ice insulates the water below, preventing lakes and rivers from freezing solid and allowing fish to survive the winter.
6. What is the difference between things that dissolve in water and things that don't?
The ability of a substance to dissolve in water is called solubility.
- Soluble substances, like salt, sugar, and lemon juice, mix completely with water and seem to disappear.
- Insoluble substances, like sand, stones, and oil, do not mix with water. No matter how much you stir them, they will either settle at the bottom (like sand) or float on top (like oil).
7. If water is clear, why do big oceans and deep lakes look blue?
That's a clever observation! While a glass of water is perfectly clear, large bodies of water appear blue due to light from the sun. Sunlight is made of many colours. Water is very good at absorbing the red and orange colours of light but tends to scatter the blue light. This scattered blue light is what reflects back to our eyes, making the deep ocean look blue. The colour can also change to green or brown if there is a lot of mud or algae in the water.









