
Why $Z{{n}^{+2}}$is diamagnetic whereas $M{{n}^{+2}}$is paramagnetic?
Answer
554.7k+ views
Hint:Magnetism is the property of a substance which depends on the pairing of electrons in them. If a substance contains no unpaired electrons and is not attracted to a magnetic field, such substances are called diamagnetic. If a substance contains unpaired electrons and is attracted to a magnetic field, then such substances are called paramagnetic substances.
Complete step-by-step answer:Let us firstly discuss $Z{{n}^{+2}}$
We know that the valence shell electronic configuration of zinc (Zn)= $\left[ Ar \right]3{{d}^{10}}4{{s}^{2}}$
We know that $Z{{n}^{+2}}$is a cation which is formed by the losing of two valence electrons by zinc (Zn)
We should remember that the electrons from the outermost subshell in the valence shell are lost by an atom. Therefore, in case of zinc electrons will be lost from the subshell.
Therefore, Electronic configuration of $Z{{n}^{+2}}=\left[ Ar \right]3{{d}^{10}}$
From the above configuration we observe that there are $10$electrons in the d subshell.
We know that in d subshell there are a total 5 orbitals. Total electrons in d subshell, is found out to be $10$. Hence, we can conclude that each orbital will have $2$electrons in it.
We also know that an orbital can have at maximum $2$electrons in it and they are paired and of opposite spin.
Hence, we can conclude that all the electrons in the d subshell are paired. This can also be represented as:
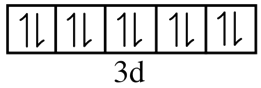
Since, all the electrons in $Z{{n}^{+2}}$are paired, it is diamagnetic.
Now let us talk about $M{{n}^{+2}}$
We know that the valence shell configuration of manganese is = $Ar\left[ 3{{d}^{5}}4{{s}^{2}} \right]$
We know that,$M{{n}^{+2}}$is a cation formed by the loss of 2 electrons from the s orbital.
Therefore, electronic configuration of valence shell of $M{{n}^{+2}}=Ar\left[ 3{{d}^{5}} \right]$
We know that there are five orbitals in d subshell and total electrons which we calculated for the manganous ion is also 5. It means one orbital will receive one electron and hence the electrons will be unpaired. This can be represented as.
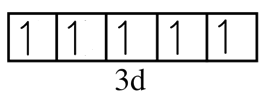
We can clearly see that electrons are unpaired in $M{{n}^{2+}}$and hence it is paramagnetic.
Note: Magnetic properties of materials are due to the magnetic moments associated with the individual electrons. Magnetic moment of each electron may originate from any of the two types of motion. Orbital motion around the nucleus or spin of electrons around its own axis.
Complete step-by-step answer:Let us firstly discuss $Z{{n}^{+2}}$
We know that the valence shell electronic configuration of zinc (Zn)= $\left[ Ar \right]3{{d}^{10}}4{{s}^{2}}$
We know that $Z{{n}^{+2}}$is a cation which is formed by the losing of two valence electrons by zinc (Zn)
We should remember that the electrons from the outermost subshell in the valence shell are lost by an atom. Therefore, in case of zinc electrons will be lost from the subshell.
Therefore, Electronic configuration of $Z{{n}^{+2}}=\left[ Ar \right]3{{d}^{10}}$
From the above configuration we observe that there are $10$electrons in the d subshell.
We know that in d subshell there are a total 5 orbitals. Total electrons in d subshell, is found out to be $10$. Hence, we can conclude that each orbital will have $2$electrons in it.
We also know that an orbital can have at maximum $2$electrons in it and they are paired and of opposite spin.
Hence, we can conclude that all the electrons in the d subshell are paired. This can also be represented as:

Since, all the electrons in $Z{{n}^{+2}}$are paired, it is diamagnetic.
Now let us talk about $M{{n}^{+2}}$
We know that the valence shell configuration of manganese is = $Ar\left[ 3{{d}^{5}}4{{s}^{2}} \right]$
We know that,$M{{n}^{+2}}$is a cation formed by the loss of 2 electrons from the s orbital.
Therefore, electronic configuration of valence shell of $M{{n}^{+2}}=Ar\left[ 3{{d}^{5}} \right]$
We know that there are five orbitals in d subshell and total electrons which we calculated for the manganous ion is also 5. It means one orbital will receive one electron and hence the electrons will be unpaired. This can be represented as.

We can clearly see that electrons are unpaired in $M{{n}^{2+}}$and hence it is paramagnetic.
Note: Magnetic properties of materials are due to the magnetic moments associated with the individual electrons. Magnetic moment of each electron may originate from any of the two types of motion. Orbital motion around the nucleus or spin of electrons around its own axis.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




