
What is Z in the following reaction sequence?
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\xrightarrow[(ii){{H}_{3}}P{{O}_{2}}+{{H}_{2}}O(iii)CO,HCl;anhydrousAlC{{l}_{3}}/CuCl]{(i)NaN{{O}_{2}}+HCl/273K}Z\]
a.) \[{{C}_{6}}{{H}_{5}}C{{O}_{2}}H\]
b.) \[{{C}_{6}}{{H}_{5}}OH\]
c.) \[{{C}_{6}}{{H}_{5}}CHO\]
d.) \[{{C}_{6}}{{H}_{6}}\]
Answer
590.1k+ views
Hint: A lot of uses are there with aniline. By using aniline we can prepare benzene diazonium chloride. Benzene diazonium chloride is the starting precursor to prepare so many compounds. The compound formed at the end of this reaction contains a carbonyl group and it gives a positive test result for tollens test and schiff's test but it gives a negative test result for the Fehling’s test.
Complete step by step answer:
The reaction given in the question contains three steps to prepare Z.
Step-1: In the first step aniline reacts with Sodium nitrate (\[NaN{{O}_{2}}\]) and hydrochloric acid (HCl) at 273K.
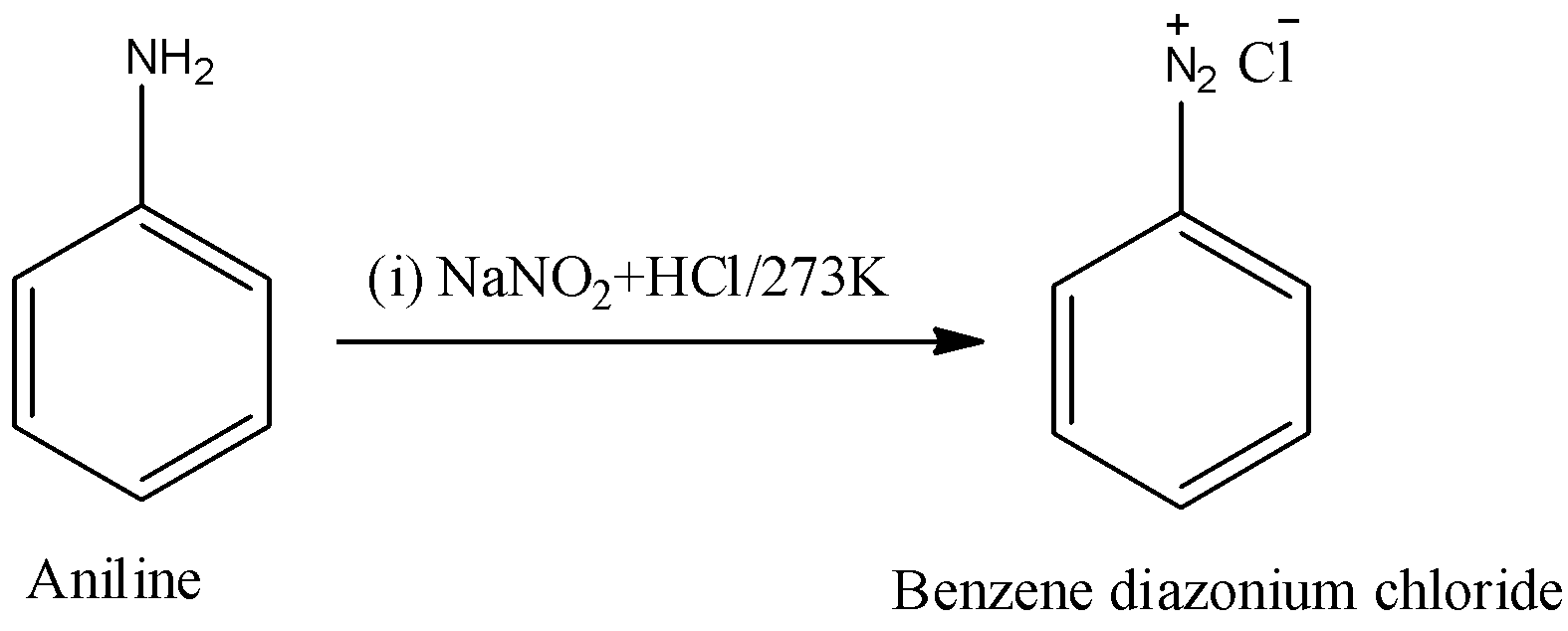
In the above reaction aniline is going to convert in to benzene diazonium chloride in the presence of Sodium nitrate (\[NaN{{O}_{2}}\]) and hydrochloric acid (HCl) at 273 K.
Step-2: The benzene diazonium chloride reacts with hypophosphorous acid and water.
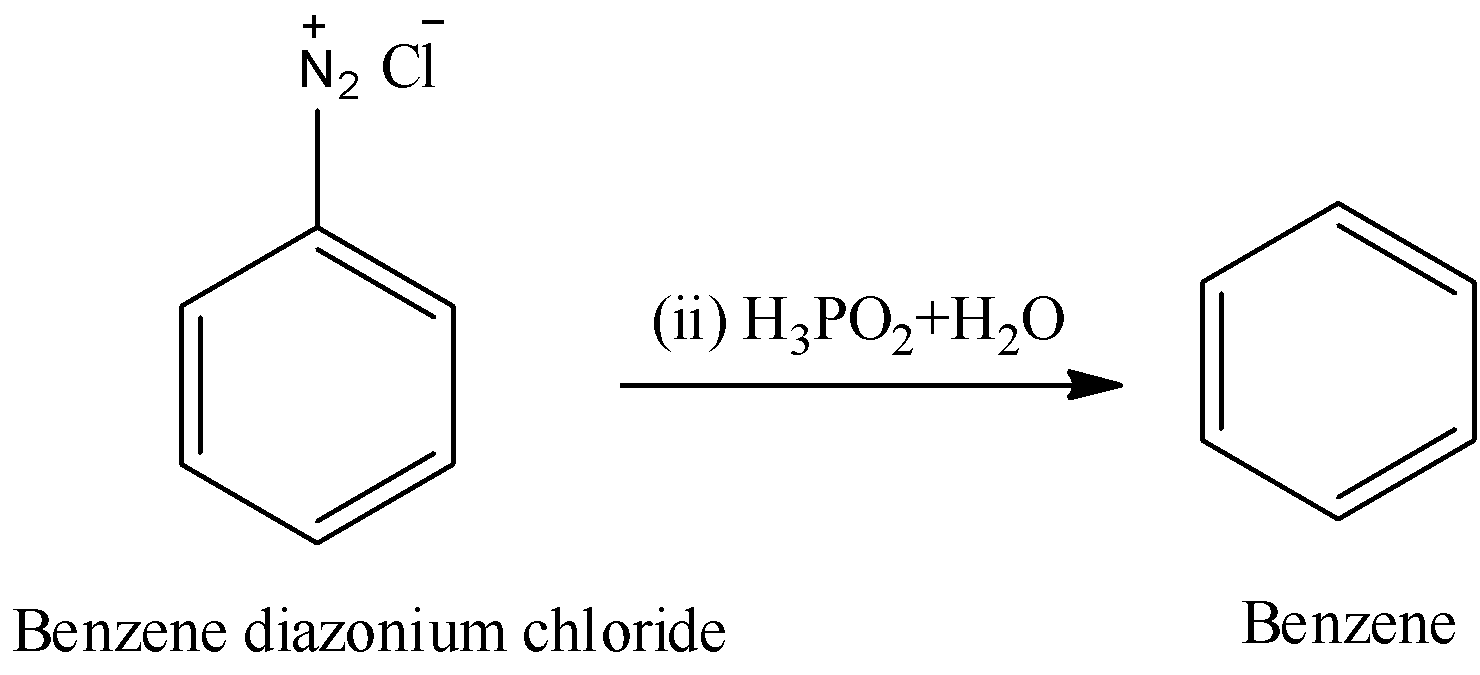
In the above reaction benzene diazonium chloride reacts with hypo phosphorous acid (\[{{H}_{3}}P{{O}_{2}}\]) and water and forms benzene as a product.
Step-3: In the third step benzene reacts with CO, HCl; anhydrous \[AlC{{l}_{3}}\]/CuCl.
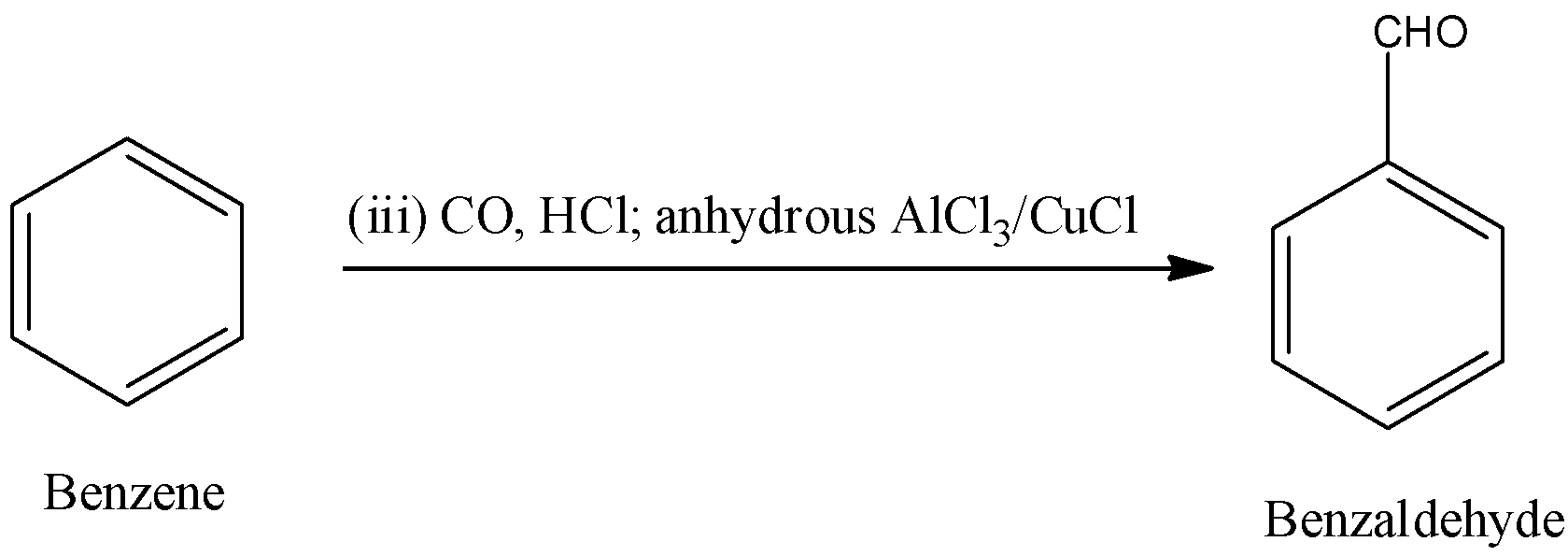
Benzene reacts with CO, HCl; anhydrous \[AlC{{l}_{3}}\]/CuCl and forms benzaldehyde as the product.
Therefore, we can write the overall reaction as follows.
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\xrightarrow[(ii){{H}_{3}}P{{O}_{2}}+{{H}_{2}}O(iii)CO,HCl;anhydrousAlC{{l}_{3}}/CuCl]{(i)NaN{{O}_{2}}+HCl/273K}{{C}_{6}}{{H}_{5}}CHO\]
Therefore the product Z is Benzaldehyde.
So, the correct answer is “Option C”.
Additional Information:
IUPAC name of benzaldehyde is benzenecarbaldehyde.
It is sometimes called benzenedicarboxaldehyde or benzoic aldehyde.
Benzaldehyde is miscible with ether, and alcohol.
Beekeepers usually apply Benzaldehyde as a bee repellent.
Benzaldehyde is commonly engaged to give almond flavor to foods.
Benzaldehyde is occasionally used in cosmetics products.
Note: By using Sandmeyer reaction we can prepare Chlorobenzene from benzene diazonium chloride.
.
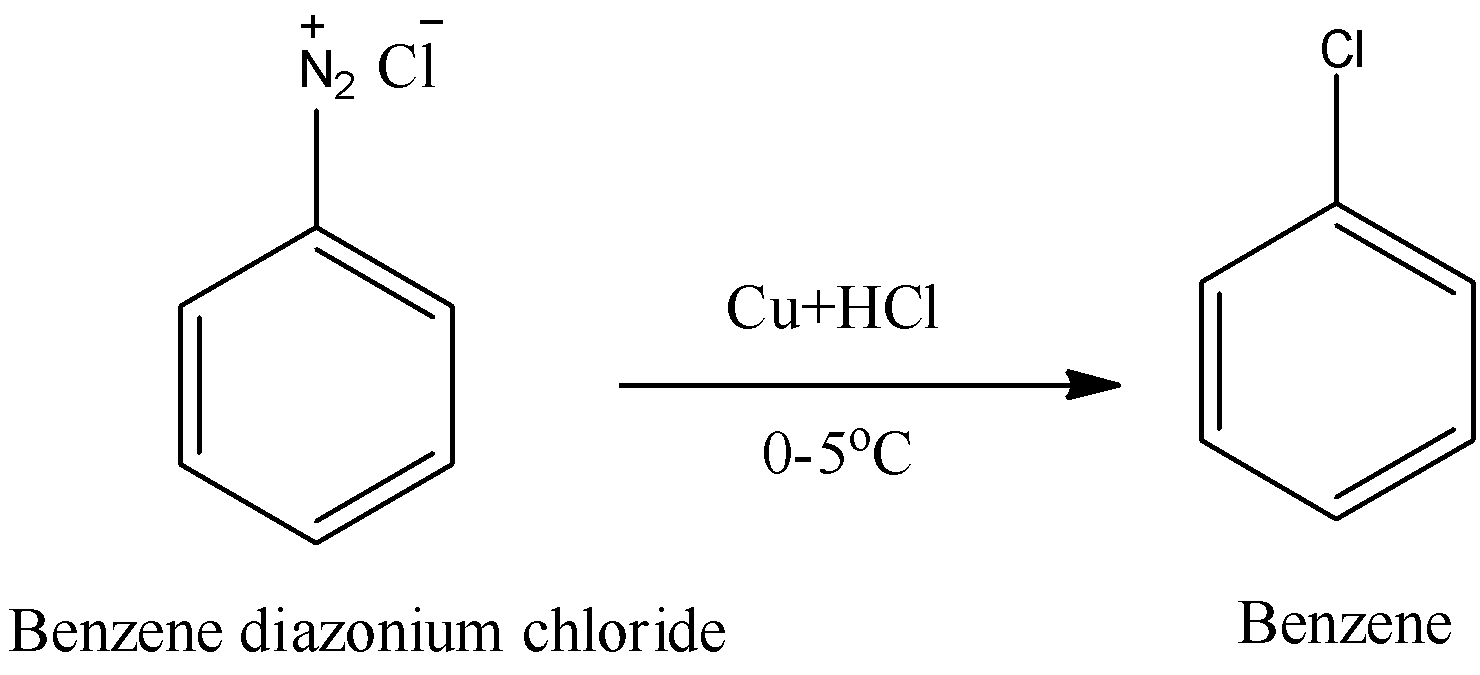
In step-2, Hypo phosphorous acid (\[{{H}_{3}}P{{O}_{2}}\]) gets oxidized to\[{{H}_{3}}P{{O}_{3}}\] and benzene is going to liberate from benzene diazonium chloride.
Complete step by step answer:
The reaction given in the question contains three steps to prepare Z.
Step-1: In the first step aniline reacts with Sodium nitrate (\[NaN{{O}_{2}}\]) and hydrochloric acid (HCl) at 273K.

In the above reaction aniline is going to convert in to benzene diazonium chloride in the presence of Sodium nitrate (\[NaN{{O}_{2}}\]) and hydrochloric acid (HCl) at 273 K.
Step-2: The benzene diazonium chloride reacts with hypophosphorous acid and water.

In the above reaction benzene diazonium chloride reacts with hypo phosphorous acid (\[{{H}_{3}}P{{O}_{2}}\]) and water and forms benzene as a product.
Step-3: In the third step benzene reacts with CO, HCl; anhydrous \[AlC{{l}_{3}}\]/CuCl.

Benzene reacts with CO, HCl; anhydrous \[AlC{{l}_{3}}\]/CuCl and forms benzaldehyde as the product.
Therefore, we can write the overall reaction as follows.
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\xrightarrow[(ii){{H}_{3}}P{{O}_{2}}+{{H}_{2}}O(iii)CO,HCl;anhydrousAlC{{l}_{3}}/CuCl]{(i)NaN{{O}_{2}}+HCl/273K}{{C}_{6}}{{H}_{5}}CHO\]
Therefore the product Z is Benzaldehyde.
So, the correct answer is “Option C”.
Additional Information:
IUPAC name of benzaldehyde is benzenecarbaldehyde.
It is sometimes called benzenedicarboxaldehyde or benzoic aldehyde.
Benzaldehyde is miscible with ether, and alcohol.
Beekeepers usually apply Benzaldehyde as a bee repellent.
Benzaldehyde is commonly engaged to give almond flavor to foods.
Benzaldehyde is occasionally used in cosmetics products.
Note: By using Sandmeyer reaction we can prepare Chlorobenzene from benzene diazonium chloride.
.

In step-2, Hypo phosphorous acid (\[{{H}_{3}}P{{O}_{2}}\]) gets oxidized to\[{{H}_{3}}P{{O}_{3}}\] and benzene is going to liberate from benzene diazonium chloride.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




