
You are given balls and stick models of six carbon atoms and fourteen hydrogen atoms and a sufficient number of sticks. In how many ways one can join the models of six carbon atoms and fourteen hydrogen atoms to form different molecules of $ {C_6}{H_{14}} $ $ ? $
Answer
507.3k+ views
Hint: Molecules can be represented in a $ 3D $ structure using various physical models to understand their structure and orientations. These are called molecular models. Ball and stick model is a molecular model in which balls are used to represent atoms and sticks represent bonds.
Complete answer:
From the hint given, we understood that balls are used to represent atoms and sticks are used to represent bonds in a ball and stick molecular model. The given compound, $ {C_6}{H_{14}} $ is an alkane with six carbon atoms and fourteen hydrogen atoms. Alkanes are hydrocarbons that have carbon-carbon and carbon-hydrogen single bonds. Their general structural formula is $ {C_n}{H_{2n + 2}} $ . Therefore, the molecule $ {C_6}{H_{14}} $ will contain nineteen sticks or single bonds.
$ {C_6}{H_{14}} $ is the molecular formula of hexane and now we have to find its number of possible structural isomers. They are:
$ 1. $
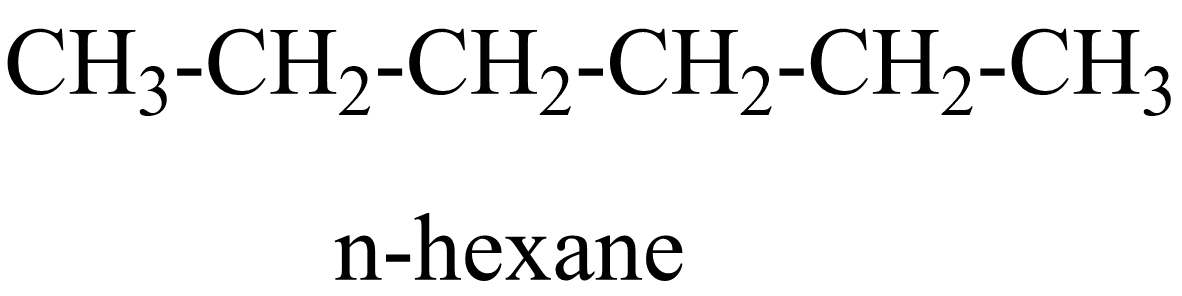
$ 2. $
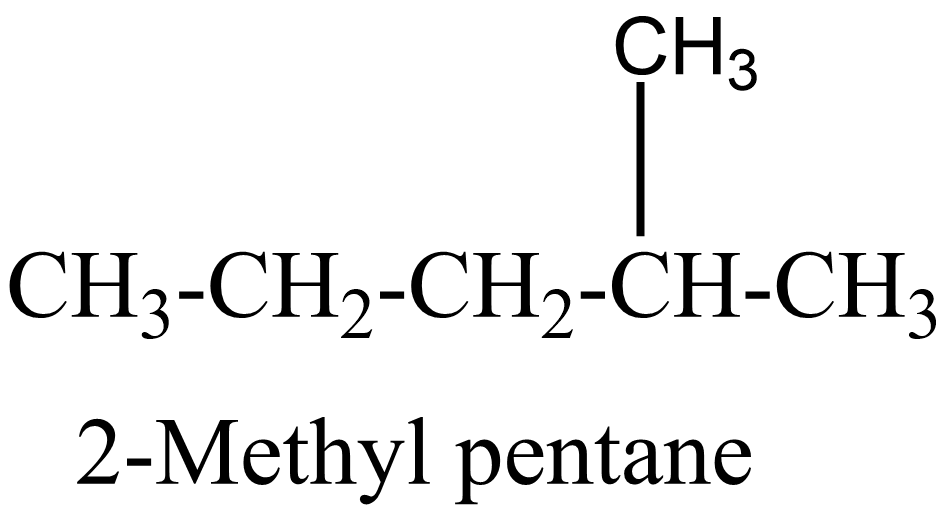
$ 3. $
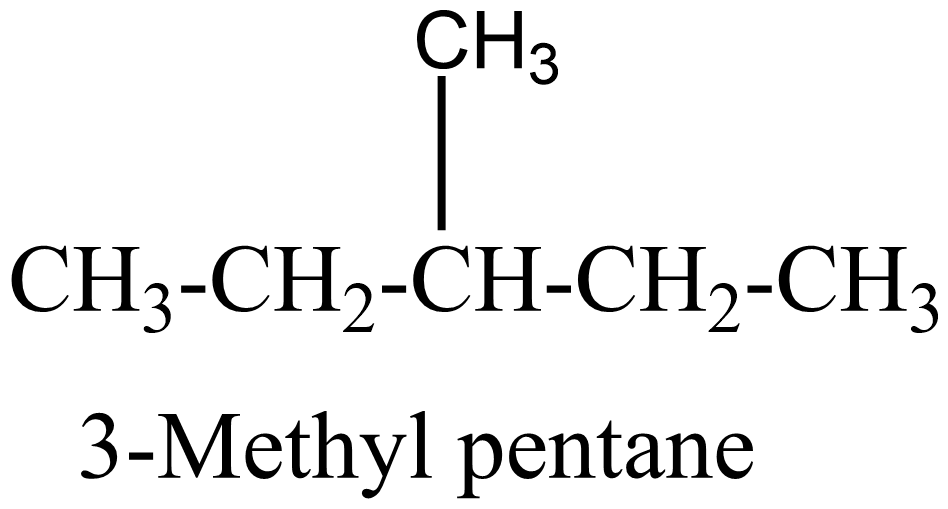
$ 4 $ .
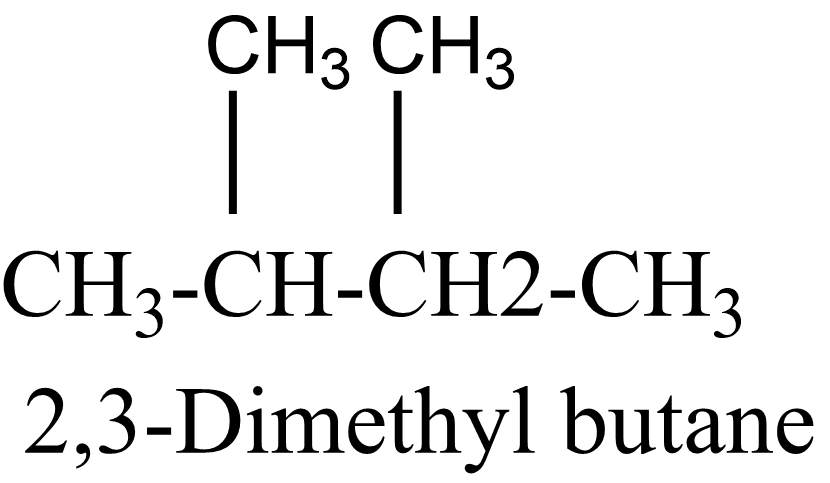
$ 5 $ .
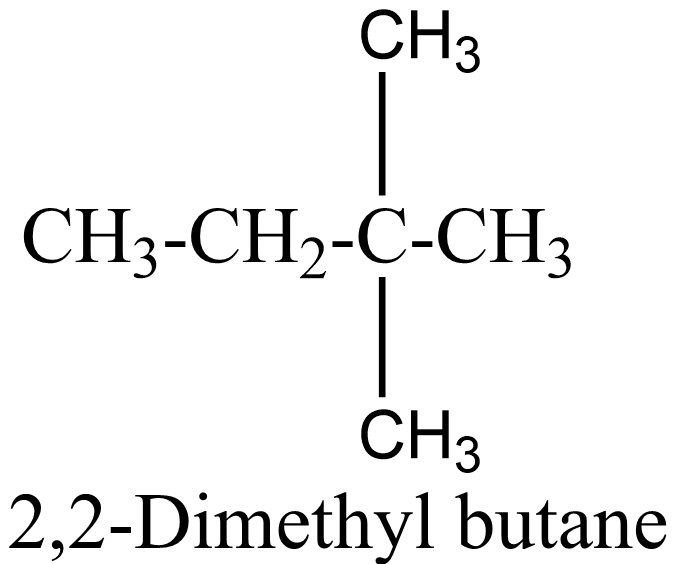
Therefore, $ {C_6}{H_{14}} $ has $ 5 $ structural isomers and thus $ 5 $ ball and stick models can be formed from the given number of balls and sticks.
In the above isomers, n-hexane is a straight chain isomer whereas others are branched chain isomers. These isomers have different physical and chemical properties due to the difference in arrangement of atoms.
Note:
Isomers are molecules with the same molecular formula but different structural formulas. They contain the same number of atoms but differ in their arrangement in space. Structural or constitutional isomerism is a form of isomerism in which bonds between the atoms are different. In stereoisomerism, bonds between the atoms are the same but their relative positions are different.
Complete answer:
From the hint given, we understood that balls are used to represent atoms and sticks are used to represent bonds in a ball and stick molecular model. The given compound, $ {C_6}{H_{14}} $ is an alkane with six carbon atoms and fourteen hydrogen atoms. Alkanes are hydrocarbons that have carbon-carbon and carbon-hydrogen single bonds. Their general structural formula is $ {C_n}{H_{2n + 2}} $ . Therefore, the molecule $ {C_6}{H_{14}} $ will contain nineteen sticks or single bonds.
$ {C_6}{H_{14}} $ is the molecular formula of hexane and now we have to find its number of possible structural isomers. They are:
$ 1. $
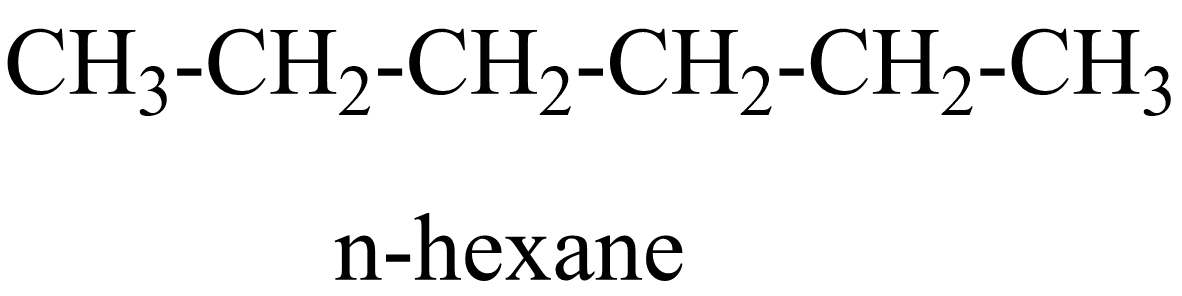
$ 2. $

$ 3. $

$ 4 $ .
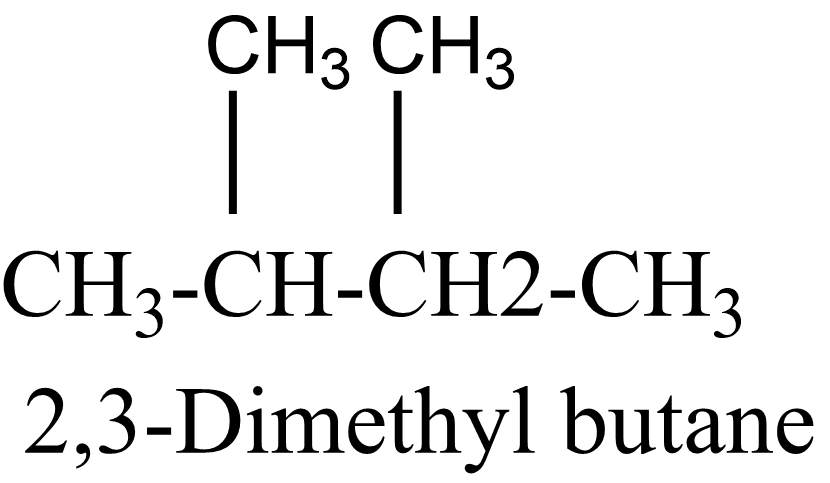
$ 5 $ .

Therefore, $ {C_6}{H_{14}} $ has $ 5 $ structural isomers and thus $ 5 $ ball and stick models can be formed from the given number of balls and sticks.
In the above isomers, n-hexane is a straight chain isomer whereas others are branched chain isomers. These isomers have different physical and chemical properties due to the difference in arrangement of atoms.
Note:
Isomers are molecules with the same molecular formula but different structural formulas. They contain the same number of atoms but differ in their arrangement in space. Structural or constitutional isomerism is a form of isomerism in which bonds between the atoms are different. In stereoisomerism, bonds between the atoms are the same but their relative positions are different.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




