
X-ray diffraction studies show that $Cu$ crystallises in a face-centred cubic unit cell with an edge $3.608 \times {10^{ - 8}}\;cm$. Another experiment shows that $Cu$ is determined to have a density of $8.92\;gc{m^{ - 3}}$. Then, what is the atomic mass of $Cu$?
Answer
514.2k+ views
Hint: The smallest part of a crystal lattice is termed a unit cell. There are three types of cubic unit cell i.e., simple cubic unit, body centred cubic and face centred cubic unit cell. In FCC unit cells, the atoms are present at each corner as well as each face centre of the cube.
Complete answer: A face centred cubic unit cell is represented as follows:
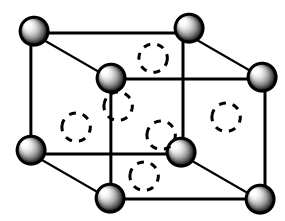
In the structure, the solid spheres denote the atoms present at the corner of the unit cell whereas the dotted sphere denotes the atoms present at the face centre of the unit cell. Total number of atoms in a single FCC unit cell are as follows:
$Z = 8 \times \dfrac{1}{8} + 6 \times \dfrac{1}{2}$
$ \Rightarrow Z = 4$
The density of a unit cell is expressed in terms of edge length, atomic mass, Avogadro’s constant and number of atoms in a unit cell as per following equation:
$\rho = \dfrac{{Z \times M}}{{{N_A} \times {a^3}}}\,\,\,\, - (i)$
Where, $\rho $ is the density of unit cell, $Z$ is the total number of atoms in a single unit cell, $M$ is the atomic mass of the element, ${N_{{A_{}}}}$is the Avogadro’s constant and $a$ is the edge length of the unit lattice.
As per question, the given data is as follows:
$a = 3.608 \times {10^{ - 8}}{\text{ cm}}$
$\rho = 8.92\;gc{m^{ - 3}}$
Substituting values in equation (i):
$8.92 = \dfrac{{4 \times M}}{{6.023 \times {{10}^{23}} \times {{\left( {3.608 \times {{10}^{ - 8}}} \right)}^3}}}$
$ \Rightarrow M = \dfrac{{2523.35 \times {{10}^{ - 1}}}}{4}$
$ \Rightarrow M = 63.08\;g$
Hence, the atomic mass of copper i.e., $Cu$ is $63.08\;g$.
Note:
It is important to note that each atom present in a unit cell does not completely contribute its each part to a single unit. The corner atoms are shared by eight-unit cells and the atoms present at the face centre are shared by two-unit cells. Hence, in the formula we always use the exact number of atoms that contribute to a cubic unit structure.
Complete answer: A face centred cubic unit cell is represented as follows:

In the structure, the solid spheres denote the atoms present at the corner of the unit cell whereas the dotted sphere denotes the atoms present at the face centre of the unit cell. Total number of atoms in a single FCC unit cell are as follows:
$Z = 8 \times \dfrac{1}{8} + 6 \times \dfrac{1}{2}$
$ \Rightarrow Z = 4$
The density of a unit cell is expressed in terms of edge length, atomic mass, Avogadro’s constant and number of atoms in a unit cell as per following equation:
$\rho = \dfrac{{Z \times M}}{{{N_A} \times {a^3}}}\,\,\,\, - (i)$
Where, $\rho $ is the density of unit cell, $Z$ is the total number of atoms in a single unit cell, $M$ is the atomic mass of the element, ${N_{{A_{}}}}$is the Avogadro’s constant and $a$ is the edge length of the unit lattice.
As per question, the given data is as follows:
$a = 3.608 \times {10^{ - 8}}{\text{ cm}}$
$\rho = 8.92\;gc{m^{ - 3}}$
Substituting values in equation (i):
$8.92 = \dfrac{{4 \times M}}{{6.023 \times {{10}^{23}} \times {{\left( {3.608 \times {{10}^{ - 8}}} \right)}^3}}}$
$ \Rightarrow M = \dfrac{{2523.35 \times {{10}^{ - 1}}}}{4}$
$ \Rightarrow M = 63.08\;g$
Hence, the atomic mass of copper i.e., $Cu$ is $63.08\;g$.
Note:
It is important to note that each atom present in a unit cell does not completely contribute its each part to a single unit. The corner atoms are shared by eight-unit cells and the atoms present at the face centre are shared by two-unit cells. Hence, in the formula we always use the exact number of atoms that contribute to a cubic unit structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




