
X, Y both respond to \[FeC{{l}_{3}}\] test and 2, 4 – DNP test. One is a thermodynamically controlled product (TCP) and the other is a kinetically controlled product (KCP). (KCP) is
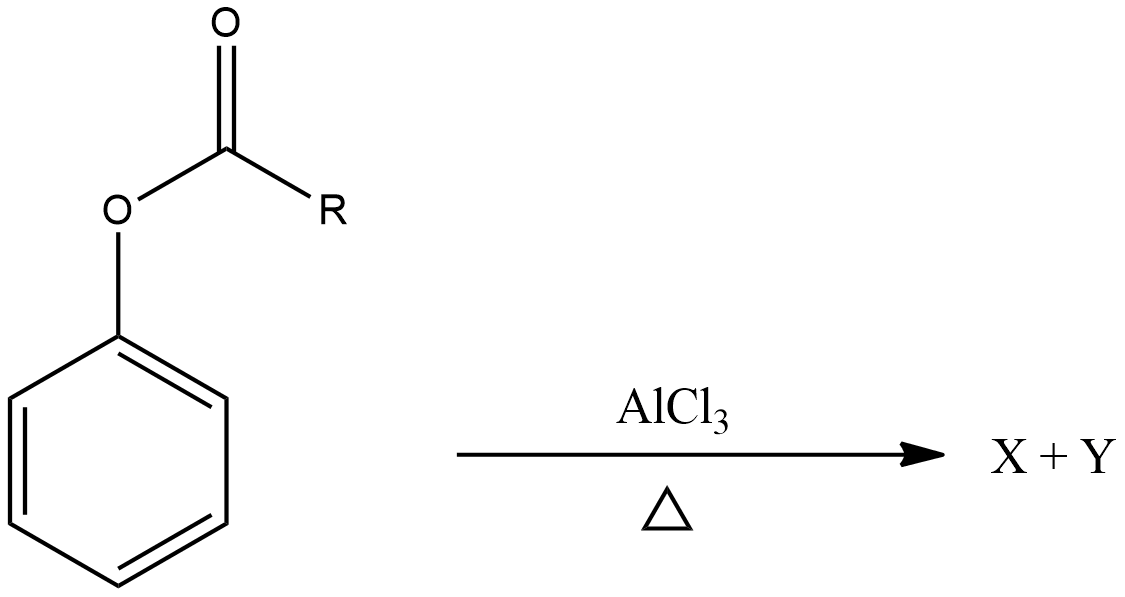
A.
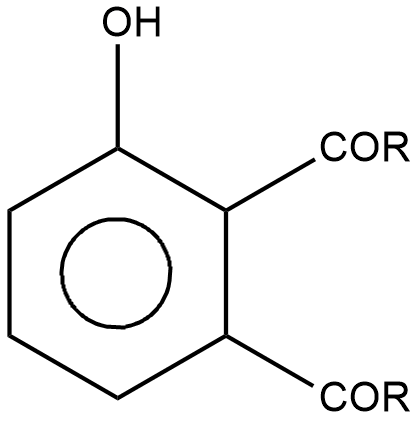
B.
C.
D.All of these.




Answer
515.4k+ views
Hint: The above given reaction is fries rearrangement, thus the products formed will be ortho and para products. Para products are less stable than ortho products, thus ortho products are formed stable.
Complete answer: The kinetically controlled product on the product side will be that of option B which is para product.
First of all, a thermodynamically controlled product is the product which is more stable and a kinetically controlled product is the product which is formed faster. Thus, the product which is formed faster in this reaction will be a kinetically controlled product.
Another reason behind the answer is intramolecular hydrogen bonding. Intramolecular hydrogen bonds are those bonds which occur within a single molecule.
The above given reaction is fries rearrangement, in this reaction an aryl ester is transformed into a hydroxy aryl ketone.
Taking the same example of our question, the fries rearrangement will go like this,
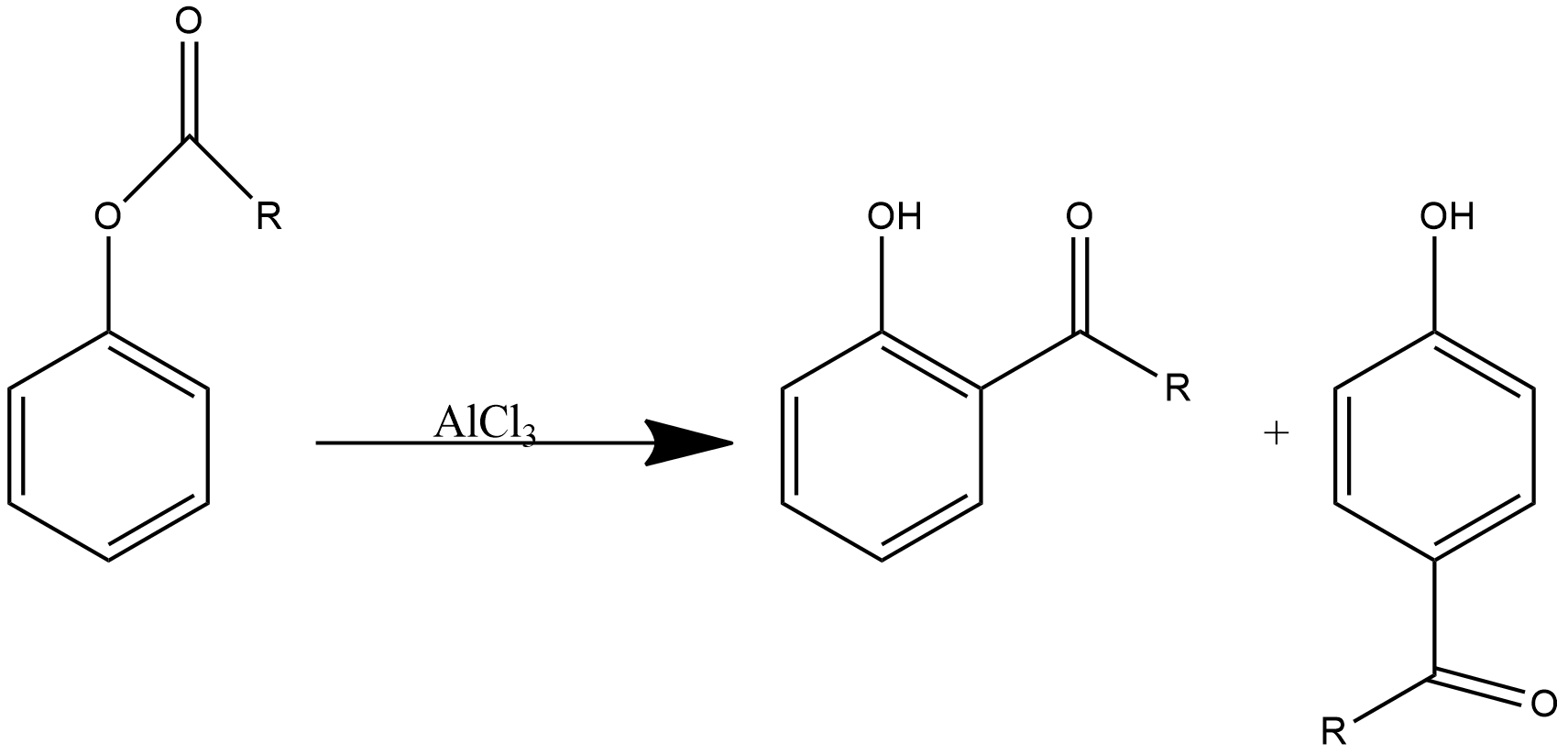
Here, an aryl ester is transformed into ortho and para products. These products are X and Y as given in the question, they are just presented in a different way. The first one is ortho and the second one is para product.
Ortho benzene products are the most stable in general nature, para products are less stable than ortho, thus para will be formed faster and ortho will be more stable.
Due to intramolecular hydrogen bonding and as ortho is more stable than para, the KCP product in the above reaction will be the para product, which is option B.
So, the answer is option B.
Note:
Both the reasons, intramolecular hydrogen bonding and the stability of ortho and para are responsible for the answer of this question. Thus, both of the reasons are important in finding the correct answer.
Complete answer: The kinetically controlled product on the product side will be that of option B which is para product.
First of all, a thermodynamically controlled product is the product which is more stable and a kinetically controlled product is the product which is formed faster. Thus, the product which is formed faster in this reaction will be a kinetically controlled product.
Another reason behind the answer is intramolecular hydrogen bonding. Intramolecular hydrogen bonds are those bonds which occur within a single molecule.
The above given reaction is fries rearrangement, in this reaction an aryl ester is transformed into a hydroxy aryl ketone.
Taking the same example of our question, the fries rearrangement will go like this,

Here, an aryl ester is transformed into ortho and para products. These products are X and Y as given in the question, they are just presented in a different way. The first one is ortho and the second one is para product.
Ortho benzene products are the most stable in general nature, para products are less stable than ortho, thus para will be formed faster and ortho will be more stable.
Due to intramolecular hydrogen bonding and as ortho is more stable than para, the KCP product in the above reaction will be the para product, which is option B.
So, the answer is option B.
Note:
Both the reasons, intramolecular hydrogen bonding and the stability of ortho and para are responsible for the answer of this question. Thus, both of the reasons are important in finding the correct answer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




