
X= number of (+M) group attached with phenyl ring, so the value of x is:
A
B
C
D
E
F
G
H








Answer
575.1k+ views
Hint: Resonance effect or mesomeric effect is defined as the flow of electrons from one part of the conjugated system that causes the creation of centres of low and high electron density. It involves the interaction of pi bonds.
Complete step by step answer:
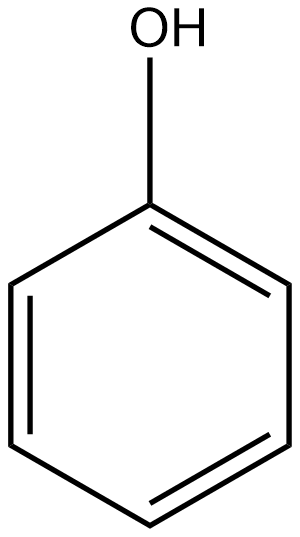
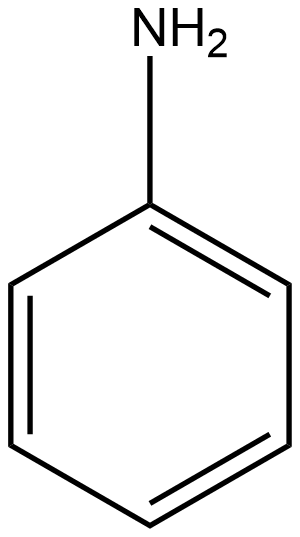
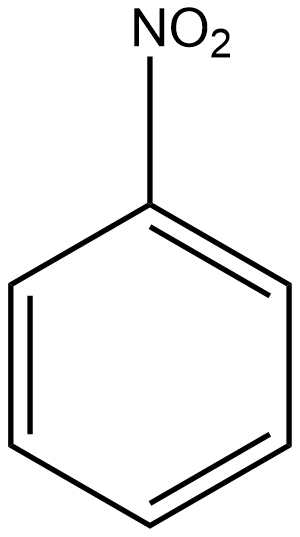
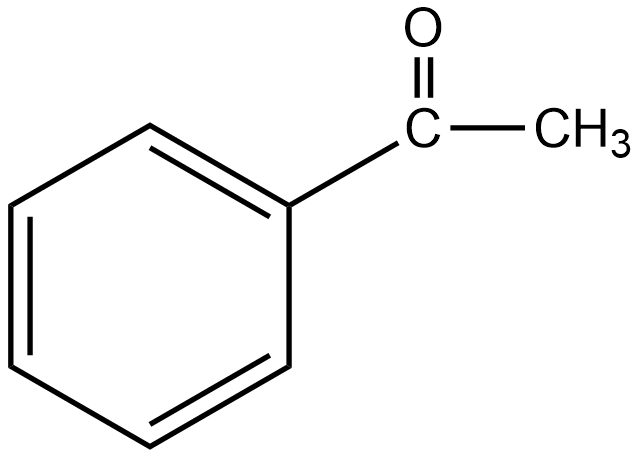
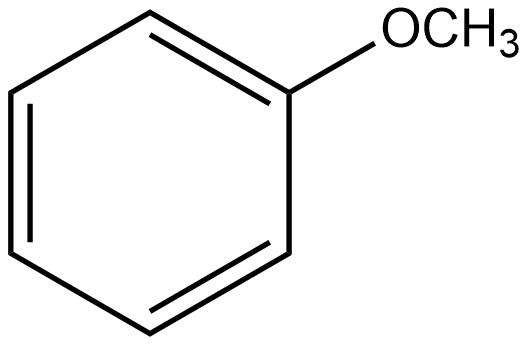
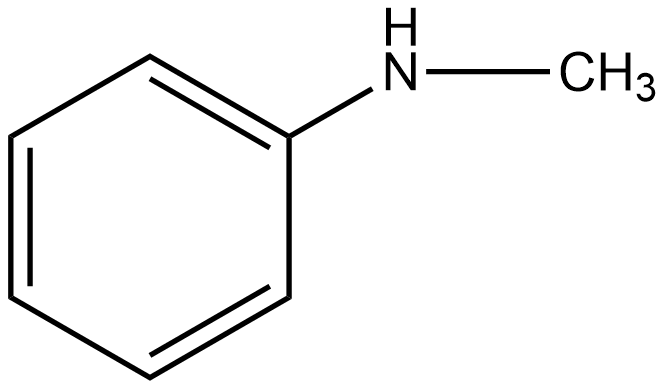
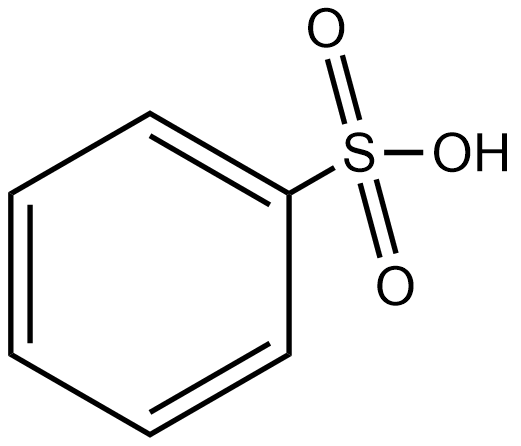
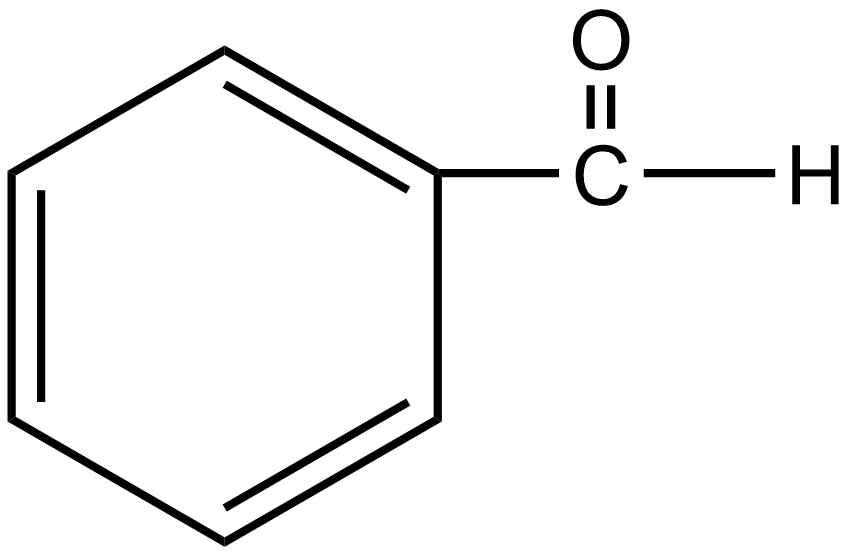
The group which donates the electrons to the double bond or a conjugated or aromatic system is said to have a +M effect. If we talk about option A i.e. phenol, since oxygen contains lone pairs of electrons hence it is an electron-donating group so it will show the +M effect. If we talk about option B i.e. amine, due to the presence of lone pairs of electrons on the nitrogen atom, it can also donate the electrons to the benzene ring so it also shows the +M effect. In the case of option C, nitrobenzene can withdraw the electrons from the benzene ring. Hence it shows –M effect. In the case of option D i.e. acetophenone, since this is an electron-withdrawing group hence it also shows –M effect. In the case of option E i.e. anisole, due to the presence of lone pair of electron on oxygen atom it also acts as an electron-donating group hence it also shows +M effect. In the case of option F, due to the presence of lone pairs on nitrogen atoms, it can donate the electrons to the aromatic ring. Hence it also shows the +M effect. In the case of option G, there is an electron-withdrawing group attached to a benzene ring, hence it will show –M effect. And in case of option H, the –CHO group is attached to the benzene ring, it acts as an electron-withdrawing group. So it shows –M effect.
Since there are four +M groups attached to the phenyl group.the value of x is 4.
So, the value of x is 4.
Note: The concept of resonance or mesomeric effect tells about the polarity induced in a molecule by the reaction between a lone pair of electrons and a pi bond. Resonance also helps to determine the stability of the compound along with the energy states.
Complete step by step answer:
The group which donates the electrons to the double bond or a conjugated or aromatic system is said to have a +M effect. If we talk about option A i.e. phenol, since oxygen contains lone pairs of electrons hence it is an electron-donating group so it will show the +M effect. If we talk about option B i.e. amine, due to the presence of lone pairs of electrons on the nitrogen atom, it can also donate the electrons to the benzene ring so it also shows the +M effect. In the case of option C, nitrobenzene can withdraw the electrons from the benzene ring. Hence it shows –M effect. In the case of option D i.e. acetophenone, since this is an electron-withdrawing group hence it also shows –M effect. In the case of option E i.e. anisole, due to the presence of lone pair of electron on oxygen atom it also acts as an electron-donating group hence it also shows +M effect. In the case of option F, due to the presence of lone pairs on nitrogen atoms, it can donate the electrons to the aromatic ring. Hence it also shows the +M effect. In the case of option G, there is an electron-withdrawing group attached to a benzene ring, hence it will show –M effect. And in case of option H, the –CHO group is attached to the benzene ring, it acts as an electron-withdrawing group. So it shows –M effect.
Since there are four +M groups attached to the phenyl group.the value of x is 4.
So, the value of x is 4.
Note: The concept of resonance or mesomeric effect tells about the polarity induced in a molecule by the reaction between a lone pair of electrons and a pi bond. Resonance also helps to determine the stability of the compound along with the energy states.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




