
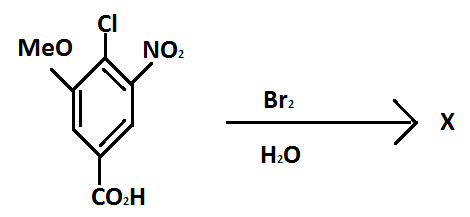
X is:


Answer
574.8k+ views
Hint:
Bromine tends to make ortho or para substitutions in benzene substituted structures. Meta position substitutions are negligible in case when bromine is taken as reagent.
Complete step by step answer:
Before approaching the solution, we should know which functional group will get the preference here. The order in decreasing order goes follows as:
\[ - COOH\]>\[ - S{O_3}H\]>\[ - COO - \]>\[ - CO(Cl)\]>\[ - CON{(H,R)_2}\]
In other terms, carboxylic group> sulfonic group> ester group> acid halide > amide.
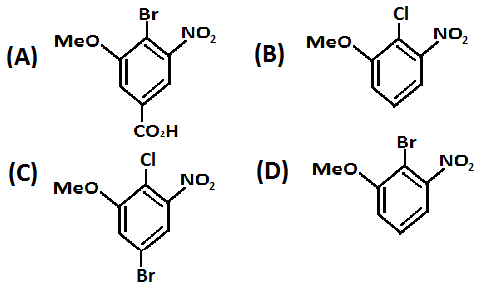
Therefore, with respect to the \[ - COOH\] group the reaction will take place. The para position chlorine will be replaced with bromine in the reaction and the product formed will be as mentioned below:
Hence, option A. is the correct option.
Additional information:
Some physical properties of bromine:
1.Bromine has an unpleasant smell.
2.It is volatile in nature, because it evaporates easily at room temperature.
3.It is three times more dense than the water itself.
4.It is the only element of group17 of periodic table that exists in liquid state.
5.Phenol decolorizes bromine water and forms 2,4,6-Tribromophenol.
6.This above mentioned reaction is the confirmation test for alcoholic functional groups.
7.The white colored precipitates of 2,4,6-Tribromophenol formed in reaction (v) shows that reaction occurs.
8.Bromine is poisonous and can cause skin burns. Thus, while performing in the lab we need to handle the reagents with care.
9.Also, it is diatomic in nature.
Note: This question is medium level. One should know the basics crystal clear so that any kind of error could be avoided. Order of preference of functional groups should be known to solve the question.
Bromine tends to make ortho or para substitutions in benzene substituted structures. Meta position substitutions are negligible in case when bromine is taken as reagent.
Complete step by step answer:
Before approaching the solution, we should know which functional group will get the preference here. The order in decreasing order goes follows as:
\[ - COOH\]>\[ - S{O_3}H\]>\[ - COO - \]>\[ - CO(Cl)\]>\[ - CON{(H,R)_2}\]
In other terms, carboxylic group> sulfonic group> ester group> acid halide > amide.
Therefore, with respect to the \[ - COOH\] group the reaction will take place. The para position chlorine will be replaced with bromine in the reaction and the product formed will be as mentioned below:
Hence, option A. is the correct option.
Additional information:
Some physical properties of bromine:
1.Bromine has an unpleasant smell.
2.It is volatile in nature, because it evaporates easily at room temperature.
3.It is three times more dense than the water itself.
4.It is the only element of group17 of periodic table that exists in liquid state.
5.Phenol decolorizes bromine water and forms 2,4,6-Tribromophenol.
6.This above mentioned reaction is the confirmation test for alcoholic functional groups.
7.The white colored precipitates of 2,4,6-Tribromophenol formed in reaction (v) shows that reaction occurs.
8.Bromine is poisonous and can cause skin burns. Thus, while performing in the lab we need to handle the reagents with care.
9.Also, it is diatomic in nature.
Note: This question is medium level. One should know the basics crystal clear so that any kind of error could be avoided. Order of preference of functional groups should be known to solve the question.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




