
Write the structures of the monomers used for getting the following polymers:
(A) Teflon
(B) Melamine-formaldehyde polymer
(C) Neoprene
Answer
592.5k+ views
Hint: A polymer is a macromolecule which is composed of a large number of small molecules. These small molecules are called monomers. These monomers are repeatedly joined together to give rise to a polymer.
Complete step by step answer:
Polymer
Polymers are the substances which are composed of macromolecules. A large number of small molecules are repeatedly joined together to form these macromolecules. These small repeating molecules are known as monomers. The method by which monomers are joined to produce a polymer is called polymerization.
So, monomers are the subunits of a polymer. Polymers can be both natural and synthetic.
A very common naturally occurring polymer is protein. On the other hand, a very common synthetic polymer is polyvinyl chloride, widely known as PVC.
a) Teflon
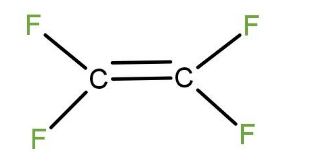
Teflon is one of the most famous synthetic polymers in the market. Teflon has been widely used to prepare non-stick kitchen appliances. Teflon is a polymer which is made by combining a large number of tetrafluroethylene molecules.
Thus, the monomer of Teflon is tetrafluroethylene molecule. The structure of tetrafluroethylene is given below:
b) Melamine formaldehyde polymer
It is another very common synthetic polymer that has a number of significant industrial usages. This polymer has a decent fire and heat resistance capability. It is very hard and durable as well. Because of these properties, this polymer is widely used in various industries.
From the name of the polymer itself, it is quite clear that this polymer is made from the condensation of two molecules melamine and formaldehyde.
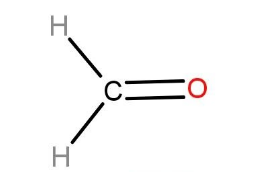
Hence, it has two monomers: melamine and formaldehyde. The structures of both the molecules are given below:
Melamine:

Formaldehyde:
c) Neoprene
Neoprene is also known as polychloroprene. This polymer is the first synthetic elastomer and it has been regulated in the market under the trade name neoprene. It is a rubber like material and has a significant resistance to oil.
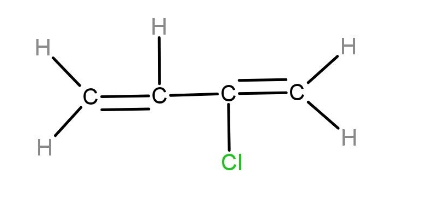
The monomer of neoprene is chloroprene. The structure of chloroprene is given below:
Note: The subunits that are joined together repeatedly to form a particular polymer are the monomers. Hence, the monomer of a polymer can be one or two or more. Hence, students should mention all the available monomers for a particular polymer.
Complete step by step answer:
Polymer
Polymers are the substances which are composed of macromolecules. A large number of small molecules are repeatedly joined together to form these macromolecules. These small repeating molecules are known as monomers. The method by which monomers are joined to produce a polymer is called polymerization.
So, monomers are the subunits of a polymer. Polymers can be both natural and synthetic.
A very common naturally occurring polymer is protein. On the other hand, a very common synthetic polymer is polyvinyl chloride, widely known as PVC.
a) Teflon
Teflon is one of the most famous synthetic polymers in the market. Teflon has been widely used to prepare non-stick kitchen appliances. Teflon is a polymer which is made by combining a large number of tetrafluroethylene molecules.
Thus, the monomer of Teflon is tetrafluroethylene molecule. The structure of tetrafluroethylene is given below:

b) Melamine formaldehyde polymer
It is another very common synthetic polymer that has a number of significant industrial usages. This polymer has a decent fire and heat resistance capability. It is very hard and durable as well. Because of these properties, this polymer is widely used in various industries.
From the name of the polymer itself, it is quite clear that this polymer is made from the condensation of two molecules melamine and formaldehyde.
Hence, it has two monomers: melamine and formaldehyde. The structures of both the molecules are given below:
Melamine:

Formaldehyde:

c) Neoprene
Neoprene is also known as polychloroprene. This polymer is the first synthetic elastomer and it has been regulated in the market under the trade name neoprene. It is a rubber like material and has a significant resistance to oil.
The monomer of neoprene is chloroprene. The structure of chloroprene is given below:

Note: The subunits that are joined together repeatedly to form a particular polymer are the monomers. Hence, the monomer of a polymer can be one or two or more. Hence, students should mention all the available monomers for a particular polymer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




