
Write the structures of the compounds A, B and C in the following reactions:
A.
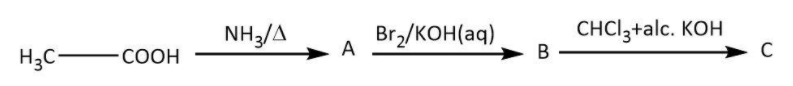
B.


Answer
574.8k+ views
Hint: We can deduce the structures of the unknown compounds by following the reactions that will take place with the given reagents in the sequential manner starting from the given reactant. Recall Hoffmann bromamide degradation reaction.
Complete step by step solution:
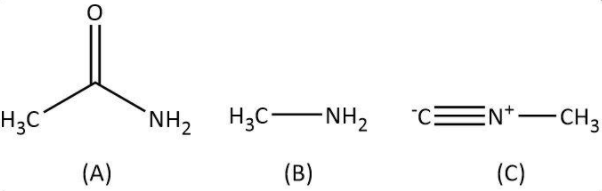
A. Let’s start with the given reactant, acetic acid. As per the question, in the first step, acetic acid has to react with ammonia followed by heating. We know that carboxylic acids reacting with ammonia give corresponding ammonium salt that on heating loses a molecule of water to give an amide. Let’s write the similar reaction for given acetic acid as follows:
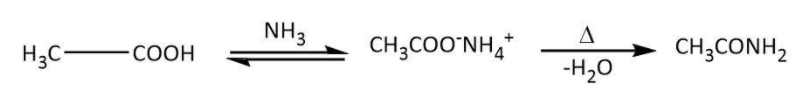
Now, we have ethanamide as our A; we will move on to the next step in which it reacts with bromine in aqueous potassium hydroxide. We have Hoffmann bromamide degradation reaction that takes place with a similar reactant (an amide) to give a primary amine having one less carbon. Let’s write the Hoffmann bromamide degradation reaction for ethanamide as follows:
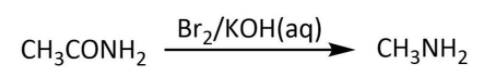
So, here we have methylamine as our compound B. Finally we will look at the last step in which our methylamine will react with chloroform and alcoholic potassium hydroxide. We have known carbylamine reaction as the one in which a primary amine upon heating with chloroform and alcoholic potassium hydroxide gives a carbylamine. Let’s write the carbylamine reaction for methylamine as follows:
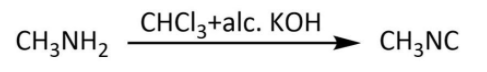
Hence, in this sequence of reactions, the structures of the compounds A, B and C are as follows:
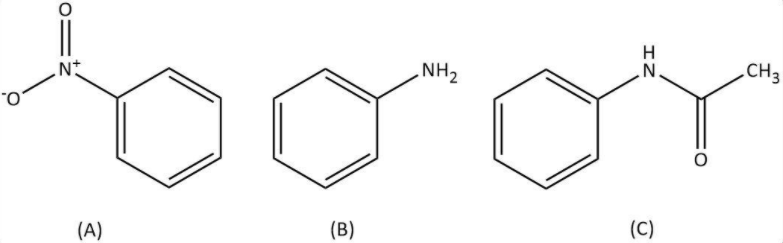
b. Let’s start with the given reactant, benzene diazonium fluoroborate. Now, as per the question, in the first step, benzene diazonium fluoroborate is heated with sodium nitrite and copper. We know that a diazonium group gets replaced by a nitro group upon heating it with sodium nitrite and copper. Let’s write a similar replacement for benzene diazonium fluoroborate as follows:
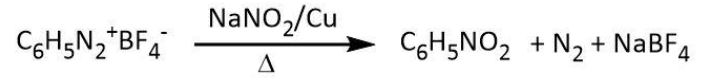
So our A compound is nitrobenzene that gets reduced in the next step with iron scrap and hydrochloric acid as follows:
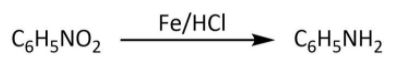
Now we have aniline as our compound B and in the last step it will get acetylated by acetyl chloride in presence of pyridine as follows:

Hence, in this sequence of reactions, the structures of the compounds A, B and C are as follows:
Note:
We can use the carbylamine reaction as a test for primary amines as it is a characteristic reaction of them and it is known as isocyanide test for primary amines.
Complete step by step solution:
A. Let’s start with the given reactant, acetic acid. As per the question, in the first step, acetic acid has to react with ammonia followed by heating. We know that carboxylic acids reacting with ammonia give corresponding ammonium salt that on heating loses a molecule of water to give an amide. Let’s write the similar reaction for given acetic acid as follows:
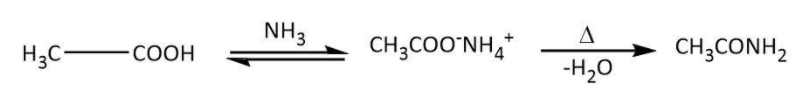
Now, we have ethanamide as our A; we will move on to the next step in which it reacts with bromine in aqueous potassium hydroxide. We have Hoffmann bromamide degradation reaction that takes place with a similar reactant (an amide) to give a primary amine having one less carbon. Let’s write the Hoffmann bromamide degradation reaction for ethanamide as follows:
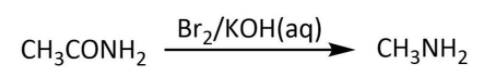
So, here we have methylamine as our compound B. Finally we will look at the last step in which our methylamine will react with chloroform and alcoholic potassium hydroxide. We have known carbylamine reaction as the one in which a primary amine upon heating with chloroform and alcoholic potassium hydroxide gives a carbylamine. Let’s write the carbylamine reaction for methylamine as follows:
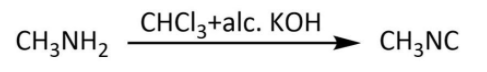
Hence, in this sequence of reactions, the structures of the compounds A, B and C are as follows:

b. Let’s start with the given reactant, benzene diazonium fluoroborate. Now, as per the question, in the first step, benzene diazonium fluoroborate is heated with sodium nitrite and copper. We know that a diazonium group gets replaced by a nitro group upon heating it with sodium nitrite and copper. Let’s write a similar replacement for benzene diazonium fluoroborate as follows:

So our A compound is nitrobenzene that gets reduced in the next step with iron scrap and hydrochloric acid as follows:
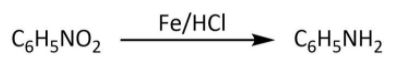
Now we have aniline as our compound B and in the last step it will get acetylated by acetyl chloride in presence of pyridine as follows:

Hence, in this sequence of reactions, the structures of the compounds A, B and C are as follows:

Note:
We can use the carbylamine reaction as a test for primary amines as it is a characteristic reaction of them and it is known as isocyanide test for primary amines.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




