
Write the structure of the main products of the following reactions:
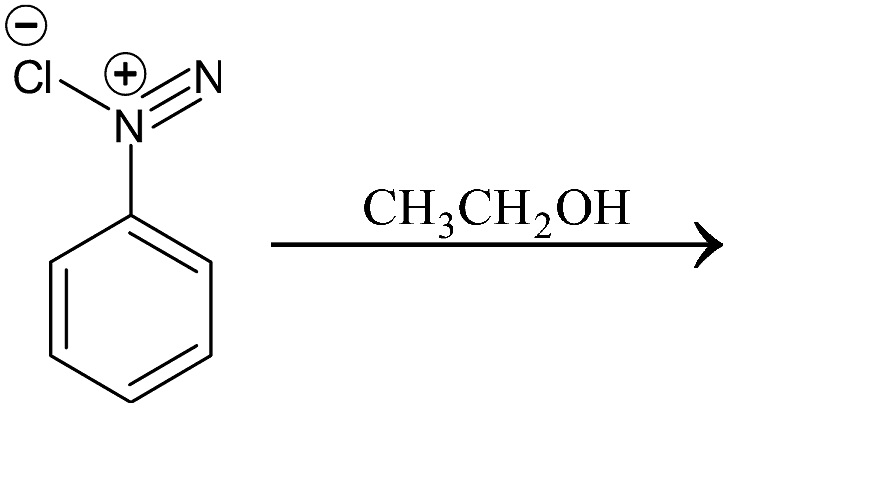

Answer
576k+ views
Hint: The given reaction is the treatment of benzene diazonium chloride with ethanol. Deamination is a reaction in which an amino group is removed as ammonia. Diazonium salt can be obtained from different ways like treating alkyl or aryl primary amine with sodium nitrite in the presence of hydrochloric acid.
Complete step by step solution:
The given reactant is an aryl diazonium salt. They are very important intermediates. In diazonium salts, over the two nitrogen atoms, the positive charge is delocalized.
In benzene diazonium chloride, $ - {{\text{N}}_2}^ + $ group is attached to the benzene ring. When the diazonium salts are involved in any reactions, then the $ - {{\text{N}}_2}^ + $ group plays a major role. It gets replaced by any other group or atom and nitrogen gas is always being evolved.
When diazonium salt is reacted with ethanol, ethanol gets oxidized to an aldehyde. And the diazonium salt will be converted as an arene. We have explained later that the nitrogen gas will be released.
The complete reaction is as shown below:
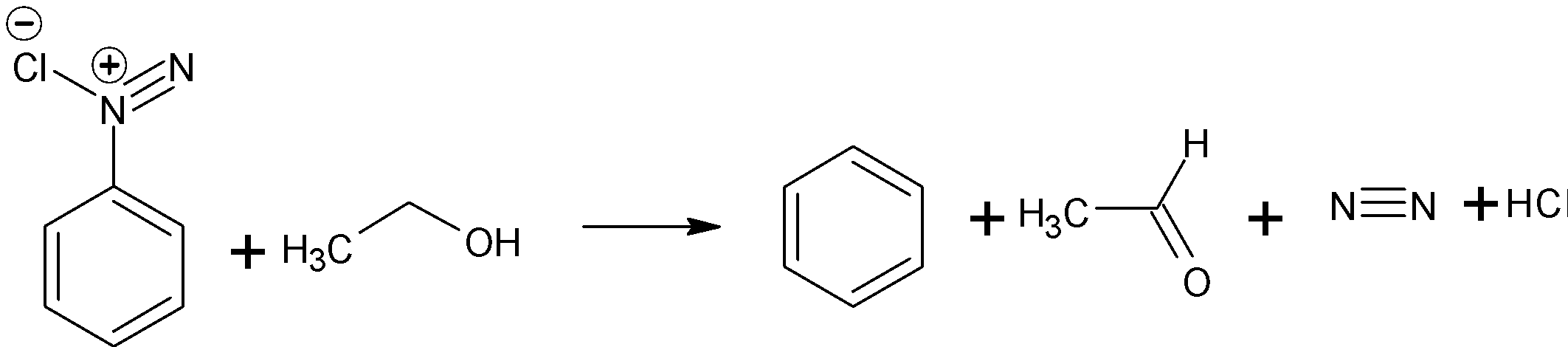
This is a type of deamination reaction. It is a nucleophilic substitution reaction.
Diazonium salt has a very good leaving group, i.e. ${{\text{N}}_2}$. This can be replaced by any nucleophiles. But in aliphatic amines, ${{\text{N}}_2}$ leaves very rapidly so that the reaction will not happen in a proper manner.
Note: Diazonium salts can be converted into different kinds of functional groups. They can be converted to aryl halide, aryl cyanide, phenol etc. When diazonium salt is reacted with ${{\text{H}}_3}{\text{P}}{{\text{O}}_2}$, it will be reduced to benzene. This reaction is an alternative for the given reaction.
Complete step by step solution:
The given reactant is an aryl diazonium salt. They are very important intermediates. In diazonium salts, over the two nitrogen atoms, the positive charge is delocalized.
In benzene diazonium chloride, $ - {{\text{N}}_2}^ + $ group is attached to the benzene ring. When the diazonium salts are involved in any reactions, then the $ - {{\text{N}}_2}^ + $ group plays a major role. It gets replaced by any other group or atom and nitrogen gas is always being evolved.
When diazonium salt is reacted with ethanol, ethanol gets oxidized to an aldehyde. And the diazonium salt will be converted as an arene. We have explained later that the nitrogen gas will be released.
The complete reaction is as shown below:

This is a type of deamination reaction. It is a nucleophilic substitution reaction.
Diazonium salt has a very good leaving group, i.e. ${{\text{N}}_2}$. This can be replaced by any nucleophiles. But in aliphatic amines, ${{\text{N}}_2}$ leaves very rapidly so that the reaction will not happen in a proper manner.
Note: Diazonium salts can be converted into different kinds of functional groups. They can be converted to aryl halide, aryl cyanide, phenol etc. When diazonium salt is reacted with ${{\text{H}}_3}{\text{P}}{{\text{O}}_2}$, it will be reduced to benzene. This reaction is an alternative for the given reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




