
Write the structure of the given compounds whose IUPAC names are given:
Ethanoyl chloride
Answer
513.9k+ views
Hint: This question is easy to solve if the rules of IUPAC nomenclature are known. Carefully look at the IUPAC name given and identify the functional groups present and identify which one will be the parent chain and if any branching is present or not.
Complete answer: Follow the IUPAC rules for drawing the structure of the compound.
According to the IUPAC nomenclature for writing the structure of the compound from the name, first identify the parent chain and write it down. Then give numbers to the carbon atoms. After that, finally, identify the functional groups attached to the parent chain and note down on which carbon the functional group is present, and write it down on the same carbon number in the structure. Lastly, cross-check the name from the structure to know whether the structure drawn matches the name or not.
Now we will analyze the given IUPAC name and follow the rules to write the structure.
The IUPAC name given is Ethanoyl chloride. It is quite clear from the name that only one alkyl group is present so the parent chain will be ethane. Next, we see that the name of ethane ends with the suffix –Oyl, -oyl suffix indicates that the functional group present in the compound is acyl chloride (a carbonyl group with chlorine at the end) which means the structure will the acyl chloride group attached to ethane molecule. Thus the structure of the IUPAC name Ethanoyl chloride is:
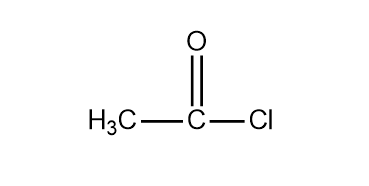
The common name of the compound is Acetyl chloride and its molecular formula is $C{H_3}COCl$.
Note:
While writing the structure it is important to check the valency of carbon as it can only have four bonds. Initially while writing the formula we can start with only writing carbon atoms, then add functional groups on the desired carbon, and then at last add hydrogens to satisfy the valency of the carbon.
Complete answer: Follow the IUPAC rules for drawing the structure of the compound.
According to the IUPAC nomenclature for writing the structure of the compound from the name, first identify the parent chain and write it down. Then give numbers to the carbon atoms. After that, finally, identify the functional groups attached to the parent chain and note down on which carbon the functional group is present, and write it down on the same carbon number in the structure. Lastly, cross-check the name from the structure to know whether the structure drawn matches the name or not.
Now we will analyze the given IUPAC name and follow the rules to write the structure.
The IUPAC name given is Ethanoyl chloride. It is quite clear from the name that only one alkyl group is present so the parent chain will be ethane. Next, we see that the name of ethane ends with the suffix –Oyl, -oyl suffix indicates that the functional group present in the compound is acyl chloride (a carbonyl group with chlorine at the end) which means the structure will the acyl chloride group attached to ethane molecule. Thus the structure of the IUPAC name Ethanoyl chloride is:

The common name of the compound is Acetyl chloride and its molecular formula is $C{H_3}COCl$.
Note:
While writing the structure it is important to check the valency of carbon as it can only have four bonds. Initially while writing the formula we can start with only writing carbon atoms, then add functional groups on the desired carbon, and then at last add hydrogens to satisfy the valency of the carbon.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




