
Write the structural formulae for the following IUPAC : methanol.
Answer
518.7k+ views
Hint: There are three key steps while writing the structural formula of any compound are Write the symbols for the elements in a chemical formula first, On these elements, write the charges (oxidation states )and the charges of the elements are crossed to each other and written as subscript in the final stage.
Complete answer:
Chemical bonds between atoms in a molecule are defined by structural formulas. A structural formula is made up of atom symbols linked by short lines that describe chemical bonds—single, double, or triple bonds are represented by one, two, or three lines, respectively.
Methanol is a transparent liquid with the chemical formula \[C{H_3}OH\]. It is also known as "wood alcohol." It's a transparent liquid with polar properties, which makes it an excellent solvent. It's also extremely flammable and, if swallowed, extremely poisonous to humans.
Structure:
Methanol is made up of a single carbon atom with a "OH" alcohol group added to it. Three hydrogen atoms occupy the remaining bonding spots on the carbon atom. The following diagram depicts this structure:
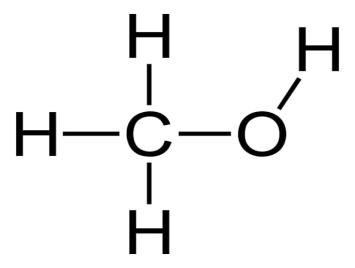
You might note that the word "methanol" has the same root as the gas "methane." In both substances, the term "meth" refers to a single carbon atom that has been saturated with hydrogen atoms. This carbon is bound to an alcohol group in "methanol," but the carbon with four hydrogens stands alone in "methane."
Methanol is similar to ethanol, also known as "grain alcohol." The alcohol used in beer, wine, and liquor is ethanol.
The prefix "eth" denotes a chain of two carbons saturated by hydrogens, while "meth" denotes a single carbon saturated by hydrogens. Ethanol, on the other hand, has a two-carbon chain, while methanol only has one.
Note:
Methanol is used to make formaldehyde, which is one of its most popular applications. This methanol-derived chemical is widely used in the manufacture of plastics, including those used in building materials, automobile parts, paints, explosives, and wrinkle-resistant synthetic fabrics. Morticians and scientists use formaldehyde to keep corpses and laboratory specimens preserved.
Complete answer:
Chemical bonds between atoms in a molecule are defined by structural formulas. A structural formula is made up of atom symbols linked by short lines that describe chemical bonds—single, double, or triple bonds are represented by one, two, or three lines, respectively.
Methanol is a transparent liquid with the chemical formula \[C{H_3}OH\]. It is also known as "wood alcohol." It's a transparent liquid with polar properties, which makes it an excellent solvent. It's also extremely flammable and, if swallowed, extremely poisonous to humans.
Structure:
Methanol is made up of a single carbon atom with a "OH" alcohol group added to it. Three hydrogen atoms occupy the remaining bonding spots on the carbon atom. The following diagram depicts this structure:

You might note that the word "methanol" has the same root as the gas "methane." In both substances, the term "meth" refers to a single carbon atom that has been saturated with hydrogen atoms. This carbon is bound to an alcohol group in "methanol," but the carbon with four hydrogens stands alone in "methane."
Methanol is similar to ethanol, also known as "grain alcohol." The alcohol used in beer, wine, and liquor is ethanol.
The prefix "eth" denotes a chain of two carbons saturated by hydrogens, while "meth" denotes a single carbon saturated by hydrogens. Ethanol, on the other hand, has a two-carbon chain, while methanol only has one.
Note:
Methanol is used to make formaldehyde, which is one of its most popular applications. This methanol-derived chemical is widely used in the manufacture of plastics, including those used in building materials, automobile parts, paints, explosives, and wrinkle-resistant synthetic fabrics. Morticians and scientists use formaldehyde to keep corpses and laboratory specimens preserved.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




