
Write the structural formula of the following: Isopropyl bromide
Answer
492.9k+ views
Hint: We need to know what is a structural formula. Organic molecules are denoted by a number of different notations. While a molecular formula only indicates the shapes and quantities of elements contained in a molecule, an extended structural notation structure may be used to define almost all of an organic material's compositional characteristics. The elemental symbols shown in the accompanying structural representation indicate the different sorts of elements that are present.
Complete answer:
We need to know that the chemical compound's structural formula is a graphical depiction of the molecular structure (derived using structural chemistry methods) that shows how the atoms could be organised in real three-dimensional space. Chemical connection inside the molecule is also demonstrated, either directly or implicitly. Unlike chemical formulae, which have a limited amount of symbols and descriptive capability, structural formulas give a more comprehensive geometric depiction of the molecular structure.
The given compound is Isopropyl bromide. The halogenated hydrocarbon with the formula \[C{H_3}CHBrC{H_3}\] is 2-bromopropane, commonly known as isopropyl bromide and 2-propyl bromide.
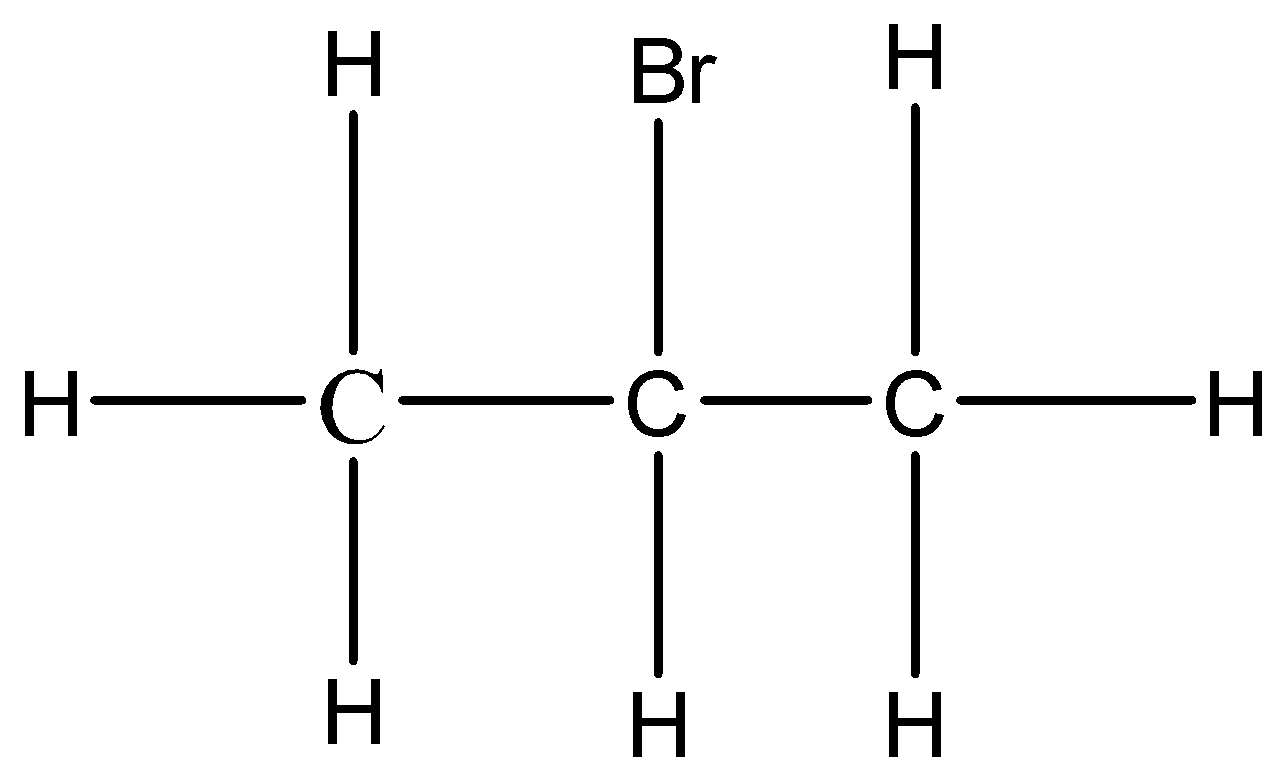
Note:
We must note that isopropyl bromide is a clear liquid with no colour. It is used in organic synthesis to introduce the isopropyl functional group. Isopropanol is heated with hydrobromic acid to produce 2-bromopropane. Because the bromine atom is in the secondary position, the molecule may readily undergo dehydrohalogenation to generate propene, which escapes as a gas and can burst closed reaction vessels. When using this reagent in base catalysed processes, potassium carbonate should be substituted for sodium or potassium hydroxide.
Complete answer:
We need to know that the chemical compound's structural formula is a graphical depiction of the molecular structure (derived using structural chemistry methods) that shows how the atoms could be organised in real three-dimensional space. Chemical connection inside the molecule is also demonstrated, either directly or implicitly. Unlike chemical formulae, which have a limited amount of symbols and descriptive capability, structural formulas give a more comprehensive geometric depiction of the molecular structure.
The given compound is Isopropyl bromide. The halogenated hydrocarbon with the formula \[C{H_3}CHBrC{H_3}\] is 2-bromopropane, commonly known as isopropyl bromide and 2-propyl bromide.

Note:
We must note that isopropyl bromide is a clear liquid with no colour. It is used in organic synthesis to introduce the isopropyl functional group. Isopropanol is heated with hydrobromic acid to produce 2-bromopropane. Because the bromine atom is in the secondary position, the molecule may readily undergo dehydrohalogenation to generate propene, which escapes as a gas and can burst closed reaction vessels. When using this reagent in base catalysed processes, potassium carbonate should be substituted for sodium or potassium hydroxide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




