
Write the structural formula of butanal.
Answer
496.5k+ views
Hint: The structural formula shows how the atoms are arranged and bonded together in a molecular formula of a chemical compound. It is the expanded form of the molecular formula. It indicates the bonding arrangement of the atoms in the molecule.
Complete answer:
The structural formula is the graphical representation of a molecular structure. A structural formula consists of symbols for the atoms connected by short lines that represent chemical bonds. It identifies the location of chemical bonds between the atoms of a molecule. Structural formulas are particularly useful for showing how compounds with the identical kind and number of atoms differ.
Now let us write the structural formula of butanal. We will follow certain steps to write the formula.
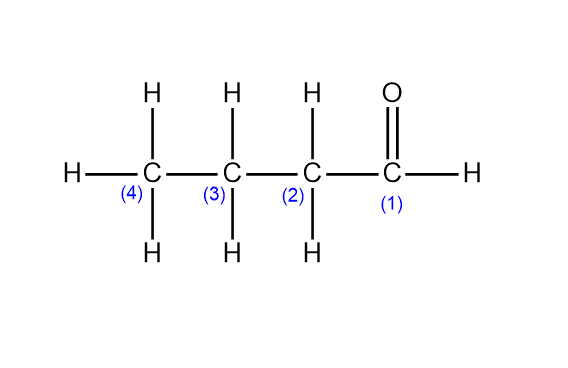
Step 1. For writing the formula, first, we need to identify the parent chain and number all the carbons present in the chain. In butanal the parent chain is butane having four carbon atoms.
Step 2. Next, identify the functional group present in the compound, and write the functional group on the carbon number which is indicated. In butanal, the functional group is an aldehyde and it seems that the aldehyde is present on carbon number one.
Step 3. By now the basic structure is complete. Now in the final step just write the number of hydrogen atoms on the carbon that satisfy their valency. One carbon can only have four bonds.
Therefore by using all the above steps the structural formula of butanal comes out be as:
Note:
The above given formula is a full structural formula. There is also something called condensed formula and skeletal structure. In a condensed formula the elements are written in a single straight line. It is exactly like writing the molecular formula. In the skeletal form, the structures of the compounds are represented by drawing a zig-zag line.
Complete answer:
The structural formula is the graphical representation of a molecular structure. A structural formula consists of symbols for the atoms connected by short lines that represent chemical bonds. It identifies the location of chemical bonds between the atoms of a molecule. Structural formulas are particularly useful for showing how compounds with the identical kind and number of atoms differ.
Now let us write the structural formula of butanal. We will follow certain steps to write the formula.
Step 1. For writing the formula, first, we need to identify the parent chain and number all the carbons present in the chain. In butanal the parent chain is butane having four carbon atoms.
Step 2. Next, identify the functional group present in the compound, and write the functional group on the carbon number which is indicated. In butanal, the functional group is an aldehyde and it seems that the aldehyde is present on carbon number one.
Step 3. By now the basic structure is complete. Now in the final step just write the number of hydrogen atoms on the carbon that satisfy their valency. One carbon can only have four bonds.
Therefore by using all the above steps the structural formula of butanal comes out be as:

Note:
The above given formula is a full structural formula. There is also something called condensed formula and skeletal structure. In a condensed formula the elements are written in a single straight line. It is exactly like writing the molecular formula. In the skeletal form, the structures of the compounds are represented by drawing a zig-zag line.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




