
Write the resonating structures for $CO_3^{2 - }$ and $HCO_3^ - $ ions.
Answer
584.1k+ views
Hint: Whenever for a molecule we can write two or more than two lewis structure but none of them is able to explain all properties of the molecule but collectively they describe all properties of that molecule is known as the phenomena of resonance and all the structures are known as resonance hybrid or conical structure.
Complete step by step answer:
There are some rules for writing the resonating structures which are discussed below:
1.The various resonating structure should differ only in the position of electrons and not in the position of atoms
2.All the resonating structures should have the same number of unpaired electrons.
3.All the resonating structure should have similar energy
The resonating structure $CO_3^{2 - }$ is given as:
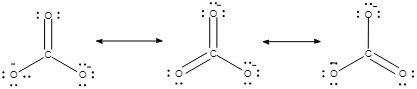
And the structure of $HCO_3^ - $ ion is given as:

Some application of resonance given below:
1.It helps to explain the ortho, para, meta directing influence of substitution in benzene.
2.It helps to explain the low reactivity of aryl and vinyl halides in nucleophilic reactions.
3.It helps to explain the acidic nature of phenols and carboxylic acid.
4.It also helps to explain the reactivity of allyl and benzyl halides.
Additional information:
Resonance measures the stability of the molecules. The greater the number of resonating structures, the more stability.
Resonance energy gives the measure of the stability of resonance hybrid, more will its value greater will be stability.
Note:
When two different resonating structures have the same number of bonds, the resonance is called isovalent resonance on the other hand if two different resonating structures have a different number of bonds then they are called heterovalent.
Complete step by step answer:
There are some rules for writing the resonating structures which are discussed below:
1.The various resonating structure should differ only in the position of electrons and not in the position of atoms
2.All the resonating structures should have the same number of unpaired electrons.
3.All the resonating structure should have similar energy
The resonating structure $CO_3^{2 - }$ is given as:

And the structure of $HCO_3^ - $ ion is given as:

Some application of resonance given below:
1.It helps to explain the ortho, para, meta directing influence of substitution in benzene.
2.It helps to explain the low reactivity of aryl and vinyl halides in nucleophilic reactions.
3.It helps to explain the acidic nature of phenols and carboxylic acid.
4.It also helps to explain the reactivity of allyl and benzyl halides.
Additional information:
Resonance measures the stability of the molecules. The greater the number of resonating structures, the more stability.
Resonance energy gives the measure of the stability of resonance hybrid, more will its value greater will be stability.
Note:
When two different resonating structures have the same number of bonds, the resonance is called isovalent resonance on the other hand if two different resonating structures have a different number of bonds then they are called heterovalent.
Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw the diagram showing the germination of pollen class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




