
Write the resonance structure of carbonate ions.
Answer
585k+ views
Hint: We know that the transfer electrons from the multiple bonds or a lone pair of electrons from an atom to another atom or an adjacent single covalent bond are called resonance.
Complete step by step answer:
We must remember that the phenomenon of the existence of a molecule in many structures due to the delocalization of electrons is defined as resonance. The resonance structures are similar in energy, bonding, and nonbonding pairs of electrons only the distribution of electrons is different. The different structures of the molecule or ion are called resonating, canonical, or contributing structures.
Let us draw different resonating structures of carbonate ions.
The resonating structure of carbonate ion is given as below,
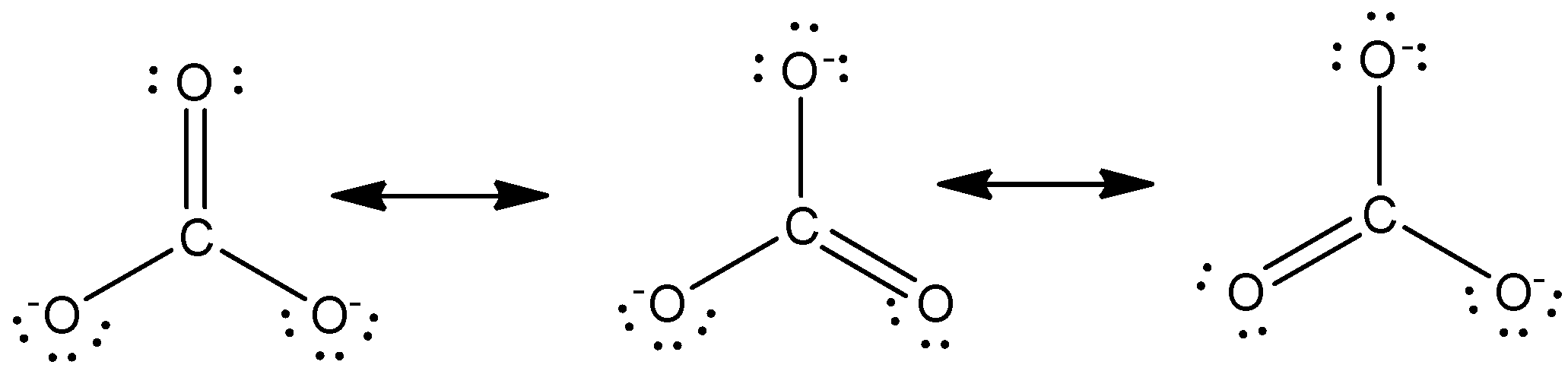
In the above structures, the central carbon atom is bonded to three oxygen atoms. As these atoms are chemically identical, therefore any of these atoms can carry a negative charge or can be bonded to the carbon atoms by a double bond. These resonating structures differ only in the distribution of electrons but otherwise the same. Hence in each resonance structure, each oxygen atom will be bonded by a double bond while the remaining two oxygen atoms will possess a negative charge.
Note:
We also know that the resonance may be a way to describe the mixture of several contributing structures into a hybrid resonance in valence bond theory in certain molecules or ions. The possibility of making mistakes is that the negative charge persists on oxygen atoms but the atoms are identical in that any of these atoms can carry a negative charge.
Complete step by step answer:
We must remember that the phenomenon of the existence of a molecule in many structures due to the delocalization of electrons is defined as resonance. The resonance structures are similar in energy, bonding, and nonbonding pairs of electrons only the distribution of electrons is different. The different structures of the molecule or ion are called resonating, canonical, or contributing structures.
Let us draw different resonating structures of carbonate ions.
The resonating structure of carbonate ion is given as below,

In the above structures, the central carbon atom is bonded to three oxygen atoms. As these atoms are chemically identical, therefore any of these atoms can carry a negative charge or can be bonded to the carbon atoms by a double bond. These resonating structures differ only in the distribution of electrons but otherwise the same. Hence in each resonance structure, each oxygen atom will be bonded by a double bond while the remaining two oxygen atoms will possess a negative charge.
Note:
We also know that the resonance may be a way to describe the mixture of several contributing structures into a hybrid resonance in valence bond theory in certain molecules or ions. The possibility of making mistakes is that the negative charge persists on oxygen atoms but the atoms are identical in that any of these atoms can carry a negative charge.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




