
Write the products of the following reactions:
i. $C{H_3} - \mathop C\limits_{}^{\mathop \parallel \limits^O } - C{H_3}\xrightarrow[{Conc.HCl}]{{Zn - Hg}}\\ $
ii. $C{H_3} - CO - Cl\xrightarrow[{Pd/BaS{O_4}}]{{{H_2}}} \\ $
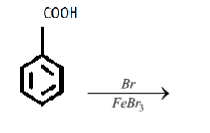
iii.

Answer
578.7k+ views
Hint:
i. The given reaction is a name reaction, and as the name suggests reduction of a carbonyl group is taking place.
ii. The given reaction is undergoing hydrogenation (addition of hydrogen) followed by reduction.
iii. The reagent in the given reaction is filled with bromine, therefore bromine atom must be added to the product molecule. But to which site?
Complete step by step solution:
i. The given reaction contains a ketone (methoxymethane) undergoing Clemmensen reduction. Where the carbonyl group is reduced to the hydrocarbon group. Now, let’s complete the reaction.
$C{H_3} - \mathop C\limits_{}^{\mathop \parallel \limits^O } - C{H_3}\xrightarrow[{Conc.HCl}]{{Zn - Hg}}{H_3}C - C{H_2} - C{H_3}$
Additional information:
1. In a Clemmensen reduction, the carbonyl group of aldehyde and ketone gets reduced to the corresponding hydrocarbon.
2. Erik Christian Clemmensen was a Danish- American chemist, known for the very reaction we just learned.
ii. In the given chemical reaction the -Cl group will get hydrogenated and the carbonyl group is reduced to aldehyde (-CHO). This is also a name reaction, known as Rosenmund reduction.
$C{H_3} - \mathop {\mathop C\limits^\parallel }\limits_{}^O - Cl\xrightarrow[{Pd/BaS{O_4}}]{{{H_2}}}{H_3}C - \mathop C\limits_{}^{\mathop \parallel \limits^O } - H$
Additional information:
1. When an acyl chloride (-C=O- Cl) is hydrogenated over a catalyst, palladium on barium sulfate. The reaction is called Rosenmund reduction.
2. Karl whelms Rosenmund was a German chemist, known for the discovery of the reaction you just solved.
iii. In the specified reaction the benzoic acid is getting brominated (bromine atom is being added). Let’s continue with the reaction;
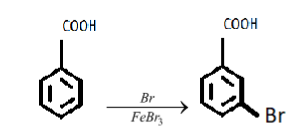
A carboxylic acid is an electron-withdrawing group(attracts the electron density from the benzene group towards itself, because of the two oxygen atoms).
Note:
i. Remember the carbonyl group reduced to the corresponding hydrocarbon in the Clemmensen reduction, and not to the corresponding alcohols.
ii. Try to avoid confusion with other reduction reactions, know that every name reaction has its unique reagents and conditions.
iii. Be careful with the reagents of bromination and Friedel-craft-alkylation, both of them have $FeBr_3$ but there is an alkyl group present in the alkylation reaction (just like its name suggests).
i. The given reaction is a name reaction, and as the name suggests reduction of a carbonyl group is taking place.
ii. The given reaction is undergoing hydrogenation (addition of hydrogen) followed by reduction.
iii. The reagent in the given reaction is filled with bromine, therefore bromine atom must be added to the product molecule. But to which site?
Complete step by step solution:
i. The given reaction contains a ketone (methoxymethane) undergoing Clemmensen reduction. Where the carbonyl group is reduced to the hydrocarbon group. Now, let’s complete the reaction.
$C{H_3} - \mathop C\limits_{}^{\mathop \parallel \limits^O } - C{H_3}\xrightarrow[{Conc.HCl}]{{Zn - Hg}}{H_3}C - C{H_2} - C{H_3}$
Additional information:
1. In a Clemmensen reduction, the carbonyl group of aldehyde and ketone gets reduced to the corresponding hydrocarbon.
2. Erik Christian Clemmensen was a Danish- American chemist, known for the very reaction we just learned.
ii. In the given chemical reaction the -Cl group will get hydrogenated and the carbonyl group is reduced to aldehyde (-CHO). This is also a name reaction, known as Rosenmund reduction.
$C{H_3} - \mathop {\mathop C\limits^\parallel }\limits_{}^O - Cl\xrightarrow[{Pd/BaS{O_4}}]{{{H_2}}}{H_3}C - \mathop C\limits_{}^{\mathop \parallel \limits^O } - H$
Additional information:
1. When an acyl chloride (-C=O- Cl) is hydrogenated over a catalyst, palladium on barium sulfate. The reaction is called Rosenmund reduction.
2. Karl whelms Rosenmund was a German chemist, known for the discovery of the reaction you just solved.
iii. In the specified reaction the benzoic acid is getting brominated (bromine atom is being added). Let’s continue with the reaction;

A carboxylic acid is an electron-withdrawing group(attracts the electron density from the benzene group towards itself, because of the two oxygen atoms).
Note:
i. Remember the carbonyl group reduced to the corresponding hydrocarbon in the Clemmensen reduction, and not to the corresponding alcohols.
ii. Try to avoid confusion with other reduction reactions, know that every name reaction has its unique reagents and conditions.
iii. Be careful with the reagents of bromination and Friedel-craft-alkylation, both of them have $FeBr_3$ but there is an alkyl group present in the alkylation reaction (just like its name suggests).
Recently Updated Pages
Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

The coating formed on the metals such as iron silver class 12 chemistry CBSE

Metals are refined by using different methods Which class 12 chemistry CBSE

What do you understand by denaturation of proteins class 12 chemistry CBSE

Assertion Nitrobenzene is used as a solvent in FriedelCrafts class 12 chemistry CBSE

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




