
Assertion
Nitrobenzene is used as a solvent in Friedel-Crafts reaction.
Reason
Fusion of p-chloronitrobenzene with KOH gives a low yield of a mixture of P-nitro phenols.
A) Both assertion and reason are correct and reason is the correct explanation for assertion.
B) Both assertion and reason are correct but reason is not the correct explanation for assertion.
C) Assertion is correct but reason is incorrect.
D) Assertion is incorrect but reason is correct.
Answer
572.4k+ views
Hint: In nitrobenzene - $N{{O}_{2}}$ group is a deactivating group.
- The electrophilic substitution cannot be done in nitrobenzene.
Complete step by step answer:
- Here in the question an assertion statement and a reason is given and we have to say which is true among the options.
- First let’s analyze the assertion statement. The assertion statement is given that the nitrobenzene is used as a solvent in Friedel-Crafts reaction. The –nitro group in nitrobenzene shows -I effect i.e. negative inductive effect, it is a ring deactivating group. Hence it attracts the electron density towards itself and decreases the electron density of the benzene ring.
- And by the mesomeric effect, the electron density in the ortho and para positions in the nitrobenzene decreases, so we cannot carry out the Friedel Crafts alkylation or acylation reaction as in the ortho or para position can’t be attacked by the electrophile formed due to decrease in the electron density.
The resonance structures of nitrobenzene are as follows-
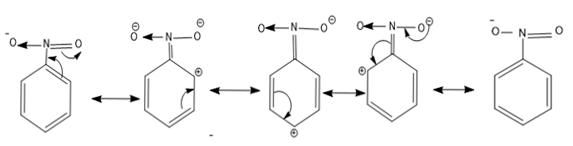
- So from the resonance structure we can say as there is a positive charge present in the ortho and para positions electrophilic attack is not possible.
So nitrobenzene is used as a solvent, the assertion statement is true.
- If the reason is taken into consideration then, it is also true because –OH group is an electrophilic group and nitrobenzene can undergo nucleophilic substitution reaction and yield a mixture of o-nitrophenol and p-nitrophenol, so that statement is also true, but assertion and reason are two independent statements. So, the correct answer is “Option B”.
Note: The inductive effect, mesomeric effect should be considered to tell about a group whether they are ring activating or deactivating by which we can confirm that electrophilic substitution takes places or nucleophilic substitution takes place. It is also important to trace the resonance structure so that we can say about the reactions that the compound can undergo which will explain the reason for the assertion statement and can find whether assertion is true or not.
- The electrophilic substitution cannot be done in nitrobenzene.
Complete step by step answer:
- Here in the question an assertion statement and a reason is given and we have to say which is true among the options.
- First let’s analyze the assertion statement. The assertion statement is given that the nitrobenzene is used as a solvent in Friedel-Crafts reaction. The –nitro group in nitrobenzene shows -I effect i.e. negative inductive effect, it is a ring deactivating group. Hence it attracts the electron density towards itself and decreases the electron density of the benzene ring.
- And by the mesomeric effect, the electron density in the ortho and para positions in the nitrobenzene decreases, so we cannot carry out the Friedel Crafts alkylation or acylation reaction as in the ortho or para position can’t be attacked by the electrophile formed due to decrease in the electron density.
The resonance structures of nitrobenzene are as follows-

- So from the resonance structure we can say as there is a positive charge present in the ortho and para positions electrophilic attack is not possible.
So nitrobenzene is used as a solvent, the assertion statement is true.
- If the reason is taken into consideration then, it is also true because –OH group is an electrophilic group and nitrobenzene can undergo nucleophilic substitution reaction and yield a mixture of o-nitrophenol and p-nitrophenol, so that statement is also true, but assertion and reason are two independent statements. So, the correct answer is “Option B”.
Note: The inductive effect, mesomeric effect should be considered to tell about a group whether they are ring activating or deactivating by which we can confirm that electrophilic substitution takes places or nucleophilic substitution takes place. It is also important to trace the resonance structure so that we can say about the reactions that the compound can undergo which will explain the reason for the assertion statement and can find whether assertion is true or not.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




