
Write the product of hydrolysis of lactose.
Answer
563.7k+ views
Hint:The hydrolysis reaction is a chemical reaction in which addition of water molecules takes place. Lactose is a disaccharide molecule consisting of small molecules.
Complete step by step answer: The hydrolysis reaction is a chemical reaction which involves rupture of bonds between two molecules using water. The water molecule in this reaction acts as a nucleophile. The carbohydrate molecule upon hydrolysis leads to formation of small sugar a molecule which is known as saccharification.
Lactose is a carbohydrate molecule which is also known as disaccharide. The chemical formula of lactose is \[{C_{12}}{H_{22}}{O_{11}}\]. The Lactose sugar is a disaccharide consisting of two monosaccharides known as galactose and glucose.
The name lactose originated from milk as lac means milk in Latin and the suffix -ose is used for labeling sugars. Naturally Lactose is present in around \[2-8\% \] of milk (by weight). The compound appears as a white solid which is water-soluble and non-hygroscopic by nature. Due to the sweet taste it is used in the food industry.
The hydrolysis reaction of lactose generates the galactose and glucose moieties. Specifically the galactose is β-D galactose and the glucose is β-D glucose. This is represented as follows:
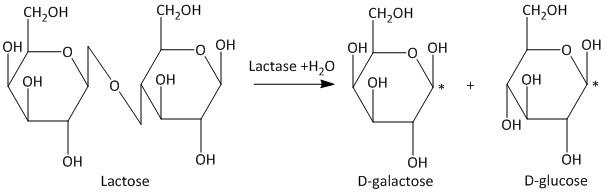
The bond which joins the two different sugar molecules is called a glycosidic bond. The position of the hydroxyl group at the anomeric carbon (marked as *) labels the sugar molecules as α and β. If the hydroxyl group is above then it is β but if the hydroxyl group is below then it is α.
Hence the product obtained by hydrolysis of lactose is β-D galactose and β-D glucose.
Note:
A Hydrolysis reaction is considered as the opposite of a condensation reaction. In condensation reaction a water molecule is lost by formation of a new bond. But in hydrolysis addition of water molecules occurs by breaking a bond.
Complete step by step answer: The hydrolysis reaction is a chemical reaction which involves rupture of bonds between two molecules using water. The water molecule in this reaction acts as a nucleophile. The carbohydrate molecule upon hydrolysis leads to formation of small sugar a molecule which is known as saccharification.
Lactose is a carbohydrate molecule which is also known as disaccharide. The chemical formula of lactose is \[{C_{12}}{H_{22}}{O_{11}}\]. The Lactose sugar is a disaccharide consisting of two monosaccharides known as galactose and glucose.
The name lactose originated from milk as lac means milk in Latin and the suffix -ose is used for labeling sugars. Naturally Lactose is present in around \[2-8\% \] of milk (by weight). The compound appears as a white solid which is water-soluble and non-hygroscopic by nature. Due to the sweet taste it is used in the food industry.
The hydrolysis reaction of lactose generates the galactose and glucose moieties. Specifically the galactose is β-D galactose and the glucose is β-D glucose. This is represented as follows:

The bond which joins the two different sugar molecules is called a glycosidic bond. The position of the hydroxyl group at the anomeric carbon (marked as *) labels the sugar molecules as α and β. If the hydroxyl group is above then it is β but if the hydroxyl group is below then it is α.
Hence the product obtained by hydrolysis of lactose is β-D galactose and β-D glucose.
Note:
A Hydrolysis reaction is considered as the opposite of a condensation reaction. In condensation reaction a water molecule is lost by formation of a new bond. But in hydrolysis addition of water molecules occurs by breaking a bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




