
Write the product of:
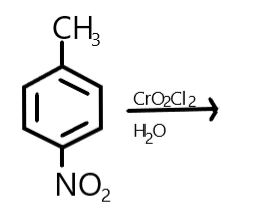

Answer
577.5k+ views
Hint: As we know that chromyl chloride is used as mild oxidant in Etard reaction involving the direct oxidation of an aromatic compound which results in the formation of an intermediate which in presence of hydronium ion converts to an aldehydic compound.
Complete Step by step answer:
- As we know that in Etard reaction oxidation of an aromatic compound results in the formation of an aldehyde in the presence of chromyl chloride and hydronium ions. For instance, a methyl benzene can be converted to benzaldehyde using chromyl chloride which acts as a mild oxidant.
- Similarly, when p-Nitrotoluene reacts with chromyl chloride in the presence of $C{S_2}$, it results in the formation of an intermediate chromium complex which on further reaction with hydronium ion results in the product formation which is generally p-nitrobenzaldehyde.
We can show this reaction as followed:
p-nitrotoluene $\xrightarrow[{C{S_2}}]{{Cr{O_2}C{l_2}}}$ chromium ion intermediate$\xrightarrow{{{H_3}{O^ + }}}$p-nitrobenzaldehyde.
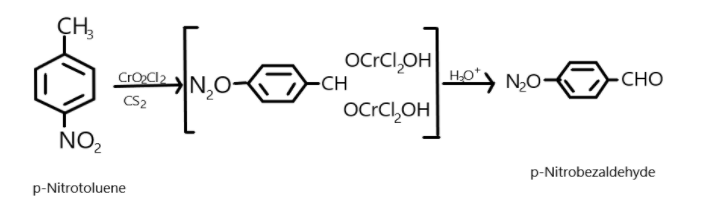
This is the easiest method to convert the aromatic nitro compounds into aromatic benzaldehyde compounds but it may be difficult to obtain the desired products from reagents as if any oxidising agents are still left in the reaction they will tend to oxidise the product formed further into a carboxylic acid compound rather than a desired aldehyde compound.
Additional information: p-Nitrobenzaldehyde is a white to yellow powder which is chiefly used in dyes and explosive materials. They are intermediate compounds of reactions including Michael addition, Henry reaction, cycloaddition, reduction and other substitution, elimination reactions.
Note: Always remember that the intermediate should be immediately decomposed by rearrangement under reducing conditions so that its further oxidation should not take place to form a carboxylic acid compound. It should be noted that in different conditions we obtain different forms of nitro benzene.
Complete Step by step answer:
- As we know that in Etard reaction oxidation of an aromatic compound results in the formation of an aldehyde in the presence of chromyl chloride and hydronium ions. For instance, a methyl benzene can be converted to benzaldehyde using chromyl chloride which acts as a mild oxidant.
- Similarly, when p-Nitrotoluene reacts with chromyl chloride in the presence of $C{S_2}$, it results in the formation of an intermediate chromium complex which on further reaction with hydronium ion results in the product formation which is generally p-nitrobenzaldehyde.
We can show this reaction as followed:
p-nitrotoluene $\xrightarrow[{C{S_2}}]{{Cr{O_2}C{l_2}}}$ chromium ion intermediate$\xrightarrow{{{H_3}{O^ + }}}$p-nitrobenzaldehyde.

This is the easiest method to convert the aromatic nitro compounds into aromatic benzaldehyde compounds but it may be difficult to obtain the desired products from reagents as if any oxidising agents are still left in the reaction they will tend to oxidise the product formed further into a carboxylic acid compound rather than a desired aldehyde compound.
Additional information: p-Nitrobenzaldehyde is a white to yellow powder which is chiefly used in dyes and explosive materials. They are intermediate compounds of reactions including Michael addition, Henry reaction, cycloaddition, reduction and other substitution, elimination reactions.
Note: Always remember that the intermediate should be immediately decomposed by rearrangement under reducing conditions so that its further oxidation should not take place to form a carboxylic acid compound. It should be noted that in different conditions we obtain different forms of nitro benzene.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




