
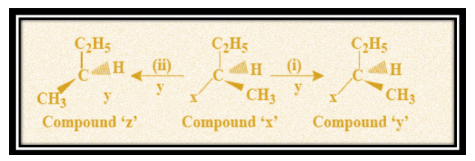
Write the names of process to obtain compound (y) and compound (z) from compound (x) in the above nucleophilic reaction (i) and (ii)?

Answer
582.6k+ views
Hint: \[{S_N}2\] reaction mechanism allows the attack of nucleophiles from the back side of the carbon atom and thus the product takes a stereochemical position opposite to the leaving group originally occupied i.e. inversion of configuration. Whereas applying \[{S_N}2\] twice will result in retention of original configuration.
Complete step by step answer:
The \[{S_N}2\] reaction is a very good example of stereospecific reaction. Stereospecific reactions are those in which different stereoisomers react to give different stereoisomers of the product. Also, \[{S_N}2\] reaction is the common example of Walden inversion in which an asymmetric carbon atom undergoes inversion of configuration.
The \[{S_N}2\] reaction is a nucleophilic substitution reaction in which a bond is broken and another bond is formed synchronously. The rate determining step of the reaction involves two reactant molecules. Since these reactions are second-order reactions, the rate-determining step depends on the concentration of the nucleophile as well as the substrate. The term \[{S_N}2\] means Substitution Nucleophilic Bimolecular reaction, also called as associative substitution and interchange mechanism.
The mechanism proceeds through a backside attack of the nucleophile on the substrate. The nucleophile approaches the substrate at an angle of 180-deg to the bond of the carbon-leaving group. This forms the carbon-nucleophile bond and simultaneously breaks the carbon-leaving group bond via a transition state.
We observe that the leaving group is pushed out of the transition state on the opposite side of the carbon-nucleophile bond, forming the desired product which has an inversion of the tetrahedral geometry at the atom in the centre.
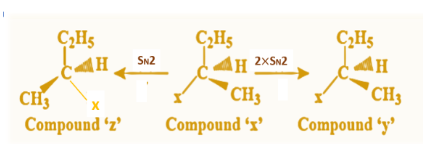
From these points, we can now conclude that double \[{S_N}2\] mechanism takes place when product ‘y’ is formed as one \[{S_N}2\] will lead to inversion and when followed by another \[{S_N}2\] process, inversion of inversion results in retention of configuration. Whereas when product ‘z’ is obtained, a single \[{S_N}2\] process occurs that gives us the product with inversion of original ‘x’ configuration.
Hence, we can say that double \[{S_N}2\] and single \[{S_N}2\] process takes place so as to obtain compound (y) and compound (z) from compound (x) in the above nucleophilic reaction (i) and (ii).
Note:
\[{S_N}2\] reactions are stereospecific i.e. not the same as stereoselective. A stereospecific mechanism specifies the stereochemical output of a reactant, whereas a stereoselective reaction selects products from those which are available by the same non-specific process acting on a given reactant.
Complete step by step answer:
The \[{S_N}2\] reaction is a very good example of stereospecific reaction. Stereospecific reactions are those in which different stereoisomers react to give different stereoisomers of the product. Also, \[{S_N}2\] reaction is the common example of Walden inversion in which an asymmetric carbon atom undergoes inversion of configuration.
The \[{S_N}2\] reaction is a nucleophilic substitution reaction in which a bond is broken and another bond is formed synchronously. The rate determining step of the reaction involves two reactant molecules. Since these reactions are second-order reactions, the rate-determining step depends on the concentration of the nucleophile as well as the substrate. The term \[{S_N}2\] means Substitution Nucleophilic Bimolecular reaction, also called as associative substitution and interchange mechanism.
The mechanism proceeds through a backside attack of the nucleophile on the substrate. The nucleophile approaches the substrate at an angle of 180-deg to the bond of the carbon-leaving group. This forms the carbon-nucleophile bond and simultaneously breaks the carbon-leaving group bond via a transition state.
We observe that the leaving group is pushed out of the transition state on the opposite side of the carbon-nucleophile bond, forming the desired product which has an inversion of the tetrahedral geometry at the atom in the centre.
From these points, we can now conclude that double \[{S_N}2\] mechanism takes place when product ‘y’ is formed as one \[{S_N}2\] will lead to inversion and when followed by another \[{S_N}2\] process, inversion of inversion results in retention of configuration. Whereas when product ‘z’ is obtained, a single \[{S_N}2\] process occurs that gives us the product with inversion of original ‘x’ configuration.

Hence, we can say that double \[{S_N}2\] and single \[{S_N}2\] process takes place so as to obtain compound (y) and compound (z) from compound (x) in the above nucleophilic reaction (i) and (ii).
Note:
\[{S_N}2\] reactions are stereospecific i.e. not the same as stereoselective. A stereospecific mechanism specifies the stereochemical output of a reactant, whereas a stereoselective reaction selects products from those which are available by the same non-specific process acting on a given reactant.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




