
Write the names and structures of monomers used for getting the following polymers.
(i) Teflon
(ii) Bakelite
Answer
582.6k+ views
Hint: Teflon is formed by the addition of simple molecule and it doesn’t involve any elimination i.e. water molecule and Bakelite is formed the molecules that a consists of the functional group and eliminates the side molecules of the reaction like water , ammonia etc. .Now, identify the monomers.
Complete step by step answer:
First of all, we should know what polymers are actually polymers. Polymers are the high molecular mass compounds, obtained by joining together a larger number of simple molecules through covalent bonds in a regular manner. And the simple molecules which combine to form a polymer are called the monomers and this process of formation of the polymers is known as the polymerization.
The above given two polymers i.e. Teflon and Bakelite are synthetic polymers which have been developed in the laboratories. Teflon is an addition polymer formed by the addition of molecules which contains the double bond and in this there is no elimination of water etc. on the other hand, Bakelite is a condensation polymer which is formed by the molecules which consists of more than one functional group and involves the elimination of water, ammonia etc.
The names and the structure of monomers which are used to get the polymers the Teflon and Bakelite are:
Note: Teflon is chemically inert substance , heat resistant and is also used as an insulator and as non- non-sticking coating on cooking utensils. On the other hand, bakelites are used in paints, varnishes, electrical goods, lacquers etc.
Complete step by step answer:
First of all, we should know what polymers are actually polymers. Polymers are the high molecular mass compounds, obtained by joining together a larger number of simple molecules through covalent bonds in a regular manner. And the simple molecules which combine to form a polymer are called the monomers and this process of formation of the polymers is known as the polymerization.
The above given two polymers i.e. Teflon and Bakelite are synthetic polymers which have been developed in the laboratories. Teflon is an addition polymer formed by the addition of molecules which contains the double bond and in this there is no elimination of water etc. on the other hand, Bakelite is a condensation polymer which is formed by the molecules which consists of more than one functional group and involves the elimination of water, ammonia etc.
The names and the structure of monomers which are used to get the polymers the Teflon and Bakelite are:
| Sr.no | polymer | Monomer | Structure |
| 1. | Teflon | tetrafluoroethylene | 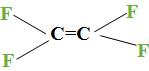
|
| 2. | Bakelite | Phenol- formaldehyde | 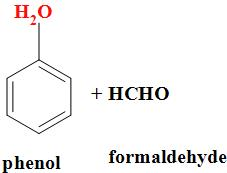
|
Note: Teflon is chemically inert substance , heat resistant and is also used as an insulator and as non- non-sticking coating on cooking utensils. On the other hand, bakelites are used in paints, varnishes, electrical goods, lacquers etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




