
Write the name the structure and the magnetic behaviour of each of the following complexes:
(i)$\left[ {{\text{Pt}}\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right){\text{Cl}}\left( {{\text{N}}{{\text{O}}_{\text{2}}}} \right)} \right]$
(ii) $\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_4}{\text{C}}{{\text{l}}_2}} \right]{\text{Cl}}$
(iii) $\left[ {{\text{Ni}}{{\left( {{\text{CO}}} \right)}_4}} \right]$
Answer
573.9k+ views
Hint: Name of compounds is written by using the IUPAC rules. According to IUPAC rules, the cation complex is named first than the anion complex. Cation and anion complex is determined by checking the charge of the complex. The presence of unpaired electrons in the metal complex tells the paramagnetic nature of the complex. The presence of all paired electrons in the metal complex tells the diamagnetic nature of the complex.
Complete Step by step answer: As per the IUPAC rules cation complex is named first then anion complex.
As per the IUPAC rules ligands are arranged in alphabetical order.
(i)
$\left[ {{\text{Pt}}\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right){\text{Cl}}\left( {{\text{N}}{{\text{O}}_{\text{2}}}} \right)} \right]$
Determine the charge of the coordination entity as follows:
Suppose the charge of the platinum is x. Charge of chloride and nitro is $ - 1$ and the charge of ammonia is zero.
$x + 0 + \,\left( { - 1} \right) + \left( { - 1} \right) = 0$
$x = + 2$
So, the charge on the platinum is $ + 2$.
The name of the $\left[ {{\text{Pt}}\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right){\text{Cl}}\left( {{\text{N}}{{\text{O}}_{\text{2}}}} \right)} \right]$ compound is as follows:
The coordination entity of platinum metal has one anionic ligand chloride named as chloro, one neutral ligand ammonia named as ammine and one anionic ligand nitro.
Ammine Dichloro Nitro Platinum(II).
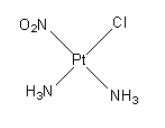
The structure of the $\left[ {{\text{Pt}}\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right){\text{Cl}}\left( {{\text{N}}{{\text{O}}_{\text{2}}}} \right)} \right]$ compound is as follows:
The coordination of platinum metal is four.
(ii)
$\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_4}{\text{C}}{{\text{l}}_2}} \right]{\text{Cl}}$
Determine the charge of the coordination entity as follows:
Suppose the charge of the cobalt is x. Charge of chloride is $ - 1$ and charge of ammonia is zero.
$x + \,\left( {0 \times 4} \right) + \left( { - 1 \times 2} \right) = + 1$
$x = + 3$
So, the charge on the cobalt is $ + 3$.
The name of the $\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_4}{\text{C}}{{\text{l}}_2}} \right]{\text{Cl}}$ compound is as follows:
The coordination entity of cobalt metal has two anionic ligand chlorides named as chloro, four neutral ligand ammonia named as ammine.
tetraamminedichlorocobalt(III).
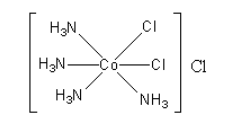
The structure of the $\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_4}{\text{C}}{{\text{l}}_2}} \right]{\text{Cl}}$ compound is as follows:
The coordination of cobalt metal is six.
(iii)
$\left[ {{\text{Ni}}{{\left( {{\text{CO}}} \right)}_4}} \right]$
Determine the charge of the coordination entity as follows:
Suppose the charge of the nickel is x. Charge of carbonyl is zero.
$x + \left( {0 \times 4} \right) = 0$
$x = 0$
So, the charge on the nickel is zero.
The name of the $\left[ {{\text{Ni}}{{\left( {{\text{CO}}} \right)}_4}} \right]$ compound is as follows:
The coordination entity of nickel metal has four neutral ligand carbonyl named as carbonyl.
Tetracarbonylnickel(0)
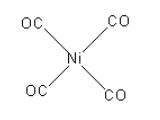
The structure of the $\left[ {{\text{Ni}}{{\left( {{\text{CO}}} \right)}_4}} \right]$ compound is as follows:
The coordination of nickel metal is four.
The magnetic property of each complex is as follows:
The splitting of d-orbital in presence of ligand field and filling of electrons is represented as follows:
Valance electronic configuration of ${\text{P}}{{\text{t}}^{2 + }}$=${\text{3}}{{\text{d}}^{10}}$.
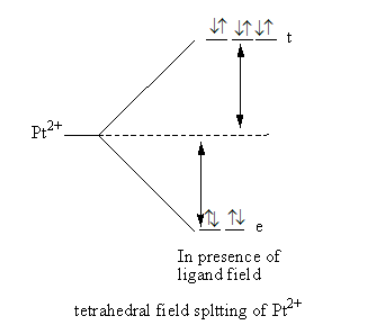
All the electrons are paired so the platinum complex is diamagnetic.
Valence electronic configuration of ${\text{C}}{{\text{o}}^{3 + }}$=${\text{3}}{{\text{d}}^6}$.
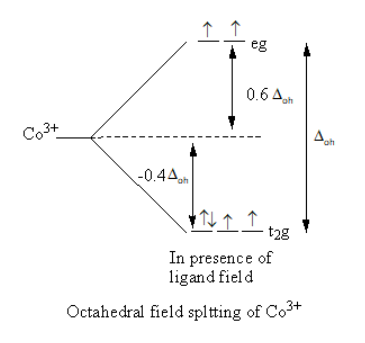
All the electrons are not paired so the cobalt complex is paramagnetic.
Valance electronic configuration of ${\text{Ni}}$=${\text{3}}{{\text{d}}^8}{\text{4}}{{\text{s}}^2}$.
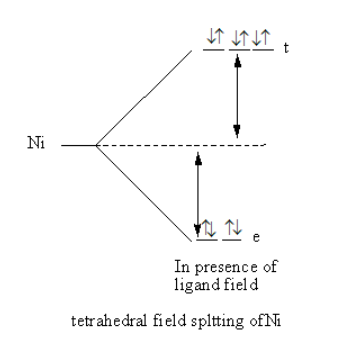
The nickel complex has no unpaired electrons so the complex is diamagnetic.
Note: The molecules that bounds with the central atom are known as ligands. Denticity defines the number of donor atoms in a ligand. The d-orbitals of the metal remain degenerate in absence of ligand field and split in presence of ligand field. For neutral ligand, the name of the compound is used as such but for water use ‘aqua’ for ammonia use ‘ammine’.
Complete Step by step answer: As per the IUPAC rules cation complex is named first then anion complex.
As per the IUPAC rules ligands are arranged in alphabetical order.
(i)
$\left[ {{\text{Pt}}\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right){\text{Cl}}\left( {{\text{N}}{{\text{O}}_{\text{2}}}} \right)} \right]$
Determine the charge of the coordination entity as follows:
Suppose the charge of the platinum is x. Charge of chloride and nitro is $ - 1$ and the charge of ammonia is zero.
$x + 0 + \,\left( { - 1} \right) + \left( { - 1} \right) = 0$
$x = + 2$
So, the charge on the platinum is $ + 2$.
The name of the $\left[ {{\text{Pt}}\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right){\text{Cl}}\left( {{\text{N}}{{\text{O}}_{\text{2}}}} \right)} \right]$ compound is as follows:
The coordination entity of platinum metal has one anionic ligand chloride named as chloro, one neutral ligand ammonia named as ammine and one anionic ligand nitro.
Ammine Dichloro Nitro Platinum(II).
The structure of the $\left[ {{\text{Pt}}\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right){\text{Cl}}\left( {{\text{N}}{{\text{O}}_{\text{2}}}} \right)} \right]$ compound is as follows:
The coordination of platinum metal is four.

(ii)
$\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_4}{\text{C}}{{\text{l}}_2}} \right]{\text{Cl}}$
Determine the charge of the coordination entity as follows:
Suppose the charge of the cobalt is x. Charge of chloride is $ - 1$ and charge of ammonia is zero.
$x + \,\left( {0 \times 4} \right) + \left( { - 1 \times 2} \right) = + 1$
$x = + 3$
So, the charge on the cobalt is $ + 3$.
The name of the $\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_4}{\text{C}}{{\text{l}}_2}} \right]{\text{Cl}}$ compound is as follows:
The coordination entity of cobalt metal has two anionic ligand chlorides named as chloro, four neutral ligand ammonia named as ammine.
tetraamminedichlorocobalt(III).
The structure of the $\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_4}{\text{C}}{{\text{l}}_2}} \right]{\text{Cl}}$ compound is as follows:
The coordination of cobalt metal is six.

(iii)
$\left[ {{\text{Ni}}{{\left( {{\text{CO}}} \right)}_4}} \right]$
Determine the charge of the coordination entity as follows:
Suppose the charge of the nickel is x. Charge of carbonyl is zero.
$x + \left( {0 \times 4} \right) = 0$
$x = 0$
So, the charge on the nickel is zero.
The name of the $\left[ {{\text{Ni}}{{\left( {{\text{CO}}} \right)}_4}} \right]$ compound is as follows:
The coordination entity of nickel metal has four neutral ligand carbonyl named as carbonyl.
Tetracarbonylnickel(0)
The structure of the $\left[ {{\text{Ni}}{{\left( {{\text{CO}}} \right)}_4}} \right]$ compound is as follows:
The coordination of nickel metal is four.

The magnetic property of each complex is as follows:
The splitting of d-orbital in presence of ligand field and filling of electrons is represented as follows:
Valance electronic configuration of ${\text{P}}{{\text{t}}^{2 + }}$=${\text{3}}{{\text{d}}^{10}}$.

All the electrons are paired so the platinum complex is diamagnetic.
Valence electronic configuration of ${\text{C}}{{\text{o}}^{3 + }}$=${\text{3}}{{\text{d}}^6}$.

All the electrons are not paired so the cobalt complex is paramagnetic.
Valance electronic configuration of ${\text{Ni}}$=${\text{3}}{{\text{d}}^8}{\text{4}}{{\text{s}}^2}$.

The nickel complex has no unpaired electrons so the complex is diamagnetic.
Note: The molecules that bounds with the central atom are known as ligands. Denticity defines the number of donor atoms in a ligand. The d-orbitals of the metal remain degenerate in absence of ligand field and split in presence of ligand field. For neutral ligand, the name of the compound is used as such but for water use ‘aqua’ for ammonia use ‘ammine’.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




