
Write the name and formula of the second member of the carbon compounds having a functional group $\text{ }-\text{OH}$.
Answer
578.1k+ views
Hint: Alcohols are the compounds that have one or more hydroxyl $\text{ }-\text{OH}$ groups bonded to aliphatic carbon atoms. According to IUPAC nomenclature, alcohols are named by replacing ‘e’ in the name of parent alkane with ‘ol’.
$\text{ Alkane }-e+ol=\text{Alkanol }$
Complete step by step solution:
The compounds obtained by replacing one hydrogen atom from an aliphatic hydrocarbon by hydroxyl group are called the alcohol.
The alcohols are represented by the general formula $\text{ ROH }$ , where R is an alkyl group.
Here, we are interested to determine the name and formula for the second member of the carbon chain having a functional group $\text{ }-\text{OH}$. The carbon chain containing one carbon atom has the word root –meth, for two carbon atoms the word root is written as -eth.
The carbon skeleton would contain two carbon atoms. The hydroxyl group would be at the carbon acquiring the first position. Thus structure would be as shown below,
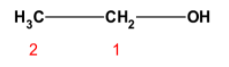
According to IUPAC nomenclature, alcohols are named by replacing ‘e’ in the name of parent alkane with ‘ol’.
$\text{ Alkane }-e+ol=\text{Alkanol }$
In this system, the following rules are followed:
1) The longest continuous chain containing the carbon bonded to $\text{ }-\text{OH}$the group is selected as the parent chain.
2) The carbon atoms in the chain are numbered in such a way that the carbon atom carrying the hydroxyl group gets the lower number.
3) The position of the substituents is indicated by a suitable number.
The word root is ‘-eth’ and the hydroxyl group is at the first carbon atom. According to IUPAC nomenclature, the name of the compound is written as,
$\text{ Ethane }-e+ol=\text{Ethanol }$
Thus, the compound of the second manner of the carbon compounds having a functional group having a functional group $\text{ }-\text{OH }$is ethanol.
Note: The alcohol has two name systems: IUPAC and common name. The common names for the may be written as the alkyl alcohol. For example, for $\text{ C}{{\text{H}}_{\text{3}}}\text{OH }$ is written as methyl alcohol, $\text{ C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{OH }$ as ethyl alcohol.
$\text{ Alkane }-e+ol=\text{Alkanol }$
Complete step by step solution:
The compounds obtained by replacing one hydrogen atom from an aliphatic hydrocarbon by hydroxyl group are called the alcohol.
The alcohols are represented by the general formula $\text{ ROH }$ , where R is an alkyl group.
Here, we are interested to determine the name and formula for the second member of the carbon chain having a functional group $\text{ }-\text{OH}$. The carbon chain containing one carbon atom has the word root –meth, for two carbon atoms the word root is written as -eth.
The carbon skeleton would contain two carbon atoms. The hydroxyl group would be at the carbon acquiring the first position. Thus structure would be as shown below,

According to IUPAC nomenclature, alcohols are named by replacing ‘e’ in the name of parent alkane with ‘ol’.
$\text{ Alkane }-e+ol=\text{Alkanol }$
In this system, the following rules are followed:
1) The longest continuous chain containing the carbon bonded to $\text{ }-\text{OH}$the group is selected as the parent chain.
2) The carbon atoms in the chain are numbered in such a way that the carbon atom carrying the hydroxyl group gets the lower number.
3) The position of the substituents is indicated by a suitable number.
The word root is ‘-eth’ and the hydroxyl group is at the first carbon atom. According to IUPAC nomenclature, the name of the compound is written as,
$\text{ Ethane }-e+ol=\text{Ethanol }$
Thus, the compound of the second manner of the carbon compounds having a functional group having a functional group $\text{ }-\text{OH }$is ethanol.
Note: The alcohol has two name systems: IUPAC and common name. The common names for the may be written as the alkyl alcohol. For example, for $\text{ C}{{\text{H}}_{\text{3}}}\text{OH }$ is written as methyl alcohol, $\text{ C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{OH }$ as ethyl alcohol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




