
Write the molecular formula and structural formula of the following substances?
A.Phosphinic acid
B.Oleum
C.Perbromic acid
Answer
571.2k+ views
Hint: We can say the molecular formula is the expression of the number of atoms present in each element in one molecule of a compound. The arrangement of the molecule is structural formula. Condensed structural formula shows the arrangement of atoms in grouped form.
Complete step by step solution:
We have to know that structural formulas identify the location of chemical bonds between the atoms of a molecule. A structural formula contains symbols for the atoms linked by short lines that indicates chemical bonds-one, two or three standing lines for single, double or triple bonds respectively.
We have to know that a molecular formula has the chemical symbols for the essential elements followed by numeric subscripts telling the number of atoms present in each element in the molecule.
A.Phosphinic acid
Phosphinic acid is nothing but hypophosphorous acid. It is an oxyacid of phosphorus that plays the role of a powerful reducing agent. In phosphinic acid, the number of hydrogen atoms is three, the number of oxygen atoms is two, and the number of phosphorus atoms is one. So, we can write the molecular formula of phosphinic acid as ${H_3}P{O_2}$. We can draw the structure of phosphinic acid as,
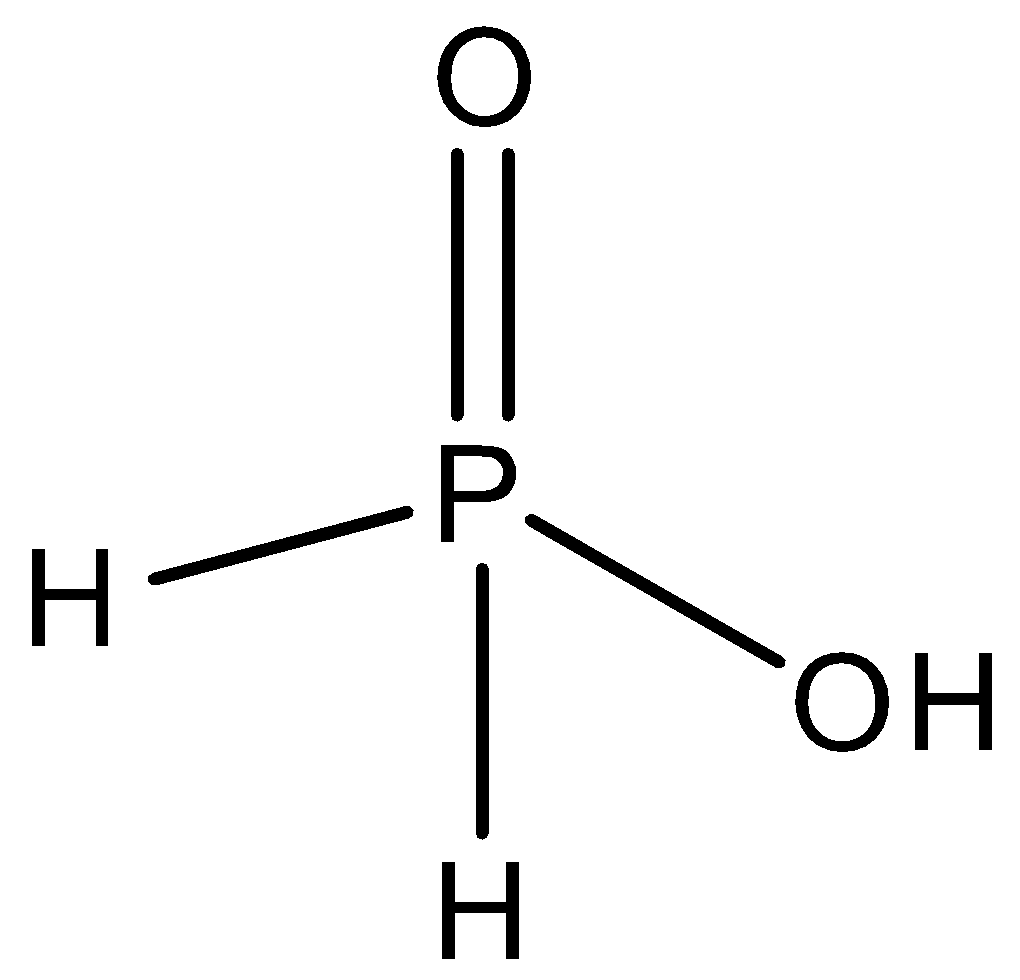
B.Oleum
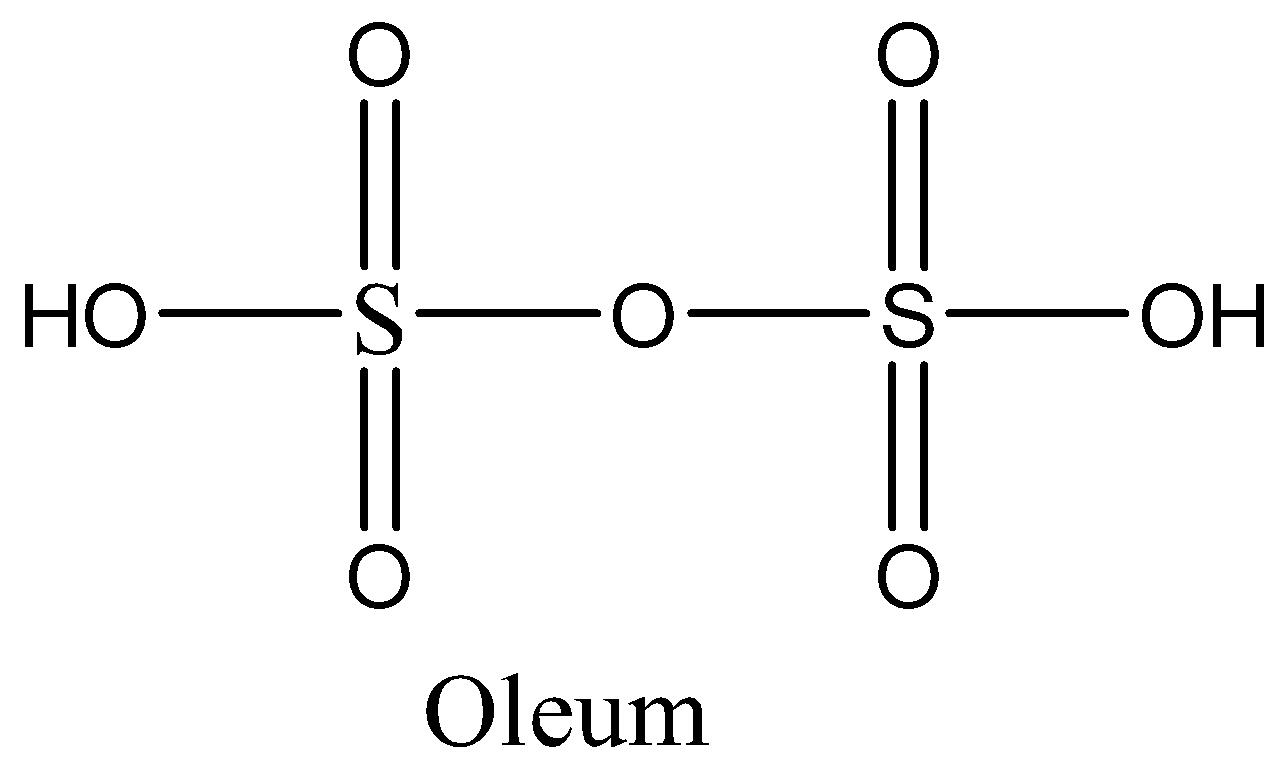
Oleum is nothing but fuming sulfuric acid. It is mineral acid and is made up of oxygen, sulfur, and hydrogen. We can write the molecular formula of oleum as ${H_2}{S_2}{O_7}$. We can draw the structure of oleum as,
C.Perbromic acid
Perbromic acid is oxyacid of bromine and it is unstable. It is a strong oxidizing agent. In perbromic acid, the number of hydrogen atoms is one, the number of oxygen atoms is four, and the number of bromine atoms is one. So, we can write the molecular formula of perbromic acid as $HBr{O_4}$. We can draw the structure of perbromic acid as,
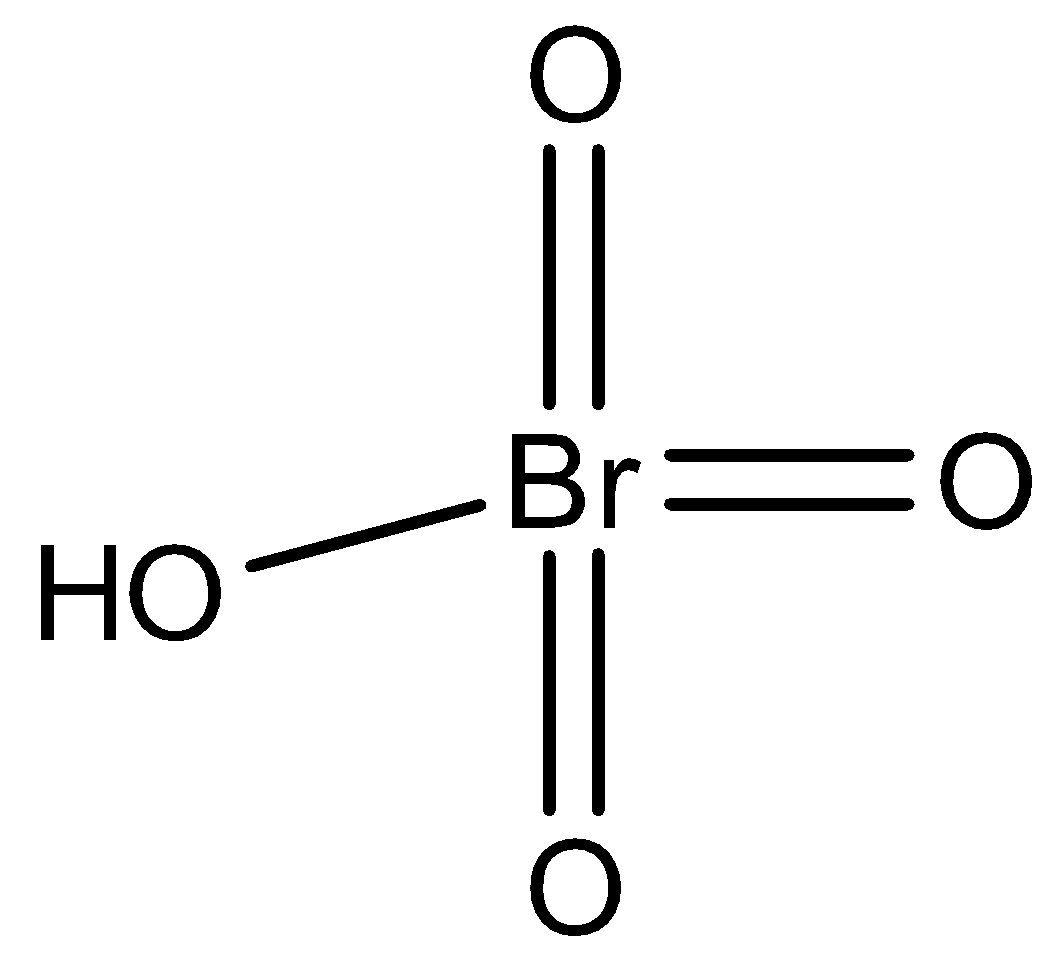
Note:One should not confuse between molecular formula, structural formula and empirical formula. An empirical formula does not mention the arrangement or number of atoms. It is typical for several ionic compounds like calcium chloride $\left( {CaC{l_2}} \right)$ , and for macromolecules such as silicon dioxide.
Complete step by step solution:
We have to know that structural formulas identify the location of chemical bonds between the atoms of a molecule. A structural formula contains symbols for the atoms linked by short lines that indicates chemical bonds-one, two or three standing lines for single, double or triple bonds respectively.
We have to know that a molecular formula has the chemical symbols for the essential elements followed by numeric subscripts telling the number of atoms present in each element in the molecule.
A.Phosphinic acid
Phosphinic acid is nothing but hypophosphorous acid. It is an oxyacid of phosphorus that plays the role of a powerful reducing agent. In phosphinic acid, the number of hydrogen atoms is three, the number of oxygen atoms is two, and the number of phosphorus atoms is one. So, we can write the molecular formula of phosphinic acid as ${H_3}P{O_2}$. We can draw the structure of phosphinic acid as,

B.Oleum
Oleum is nothing but fuming sulfuric acid. It is mineral acid and is made up of oxygen, sulfur, and hydrogen. We can write the molecular formula of oleum as ${H_2}{S_2}{O_7}$. We can draw the structure of oleum as,

C.Perbromic acid
Perbromic acid is oxyacid of bromine and it is unstable. It is a strong oxidizing agent. In perbromic acid, the number of hydrogen atoms is one, the number of oxygen atoms is four, and the number of bromine atoms is one. So, we can write the molecular formula of perbromic acid as $HBr{O_4}$. We can draw the structure of perbromic acid as,

Note:One should not confuse between molecular formula, structural formula and empirical formula. An empirical formula does not mention the arrangement or number of atoms. It is typical for several ionic compounds like calcium chloride $\left( {CaC{l_2}} \right)$ , and for macromolecules such as silicon dioxide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




