
Write the mechanism of nucleophilic substitution, when p-nitro chlorobenzene is treated with caustic soda.
Answer
580.2k+ views
Hint: Reactions involving replacement of halo group of aryl halide with some other substituent is known as nucleophilic aromatic substitution reaction. Aryl halides are less reactive than alkyl halides towards nucleophilic substitution reaction.
Complete step by step solution:
We know that aryl halides are resonance stabilized due to delocalization of electrons, so that $C - X$bond acquires some double bond character which makes its cleavage difficult.
Thus aryl halides undergo nucleophilic substitution reaction at high temperature and pressure only.
Now let us look into the mechanism of the reaction
We have caustic soda which is the $NaOH$, the $O{H^ - }$of caustic soda attacks the chlorine part in the benzene
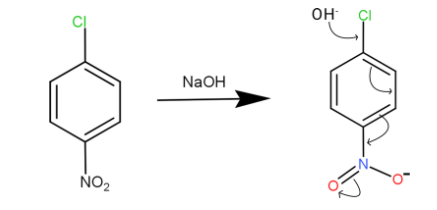
And when this happens due to resonating structure and the electron withdrawing group shift the electrons to accommodate the $O{H^ - }$. We can see the bond shift marked by the arrows.
This compound is very unstable and it again goes to its resonating structure
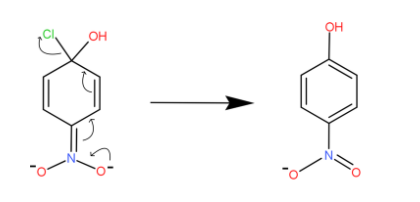
The electrons on the one side of nitro pass into its nitrogen bond and it circulates throughout the structure. We can see the movement of electrons or bonds by arrow marks. At last the bond on the chlorine atom shifts towards it and it gets detached.
Finally, we have the chlorine atom detached and hydroxide gets attached to that position. This is how the halo group is replaced in aryl halides.
Note: Here apart from chlorine attached to the benzene group we have an electron withdrawing group $ - N{O_2}$ at the para position. Presence of a strong electron withdrawing group at ortho or para position enhances the reactivity of aryl halides towards nucleophilic substitution.
We can also say that with the presence of an electron withdrawing group the reaction can occur in less temperature and pressure.
Complete step by step solution:
We know that aryl halides are resonance stabilized due to delocalization of electrons, so that $C - X$bond acquires some double bond character which makes its cleavage difficult.
Thus aryl halides undergo nucleophilic substitution reaction at high temperature and pressure only.
Now let us look into the mechanism of the reaction
We have caustic soda which is the $NaOH$, the $O{H^ - }$of caustic soda attacks the chlorine part in the benzene

And when this happens due to resonating structure and the electron withdrawing group shift the electrons to accommodate the $O{H^ - }$. We can see the bond shift marked by the arrows.
This compound is very unstable and it again goes to its resonating structure

The electrons on the one side of nitro pass into its nitrogen bond and it circulates throughout the structure. We can see the movement of electrons or bonds by arrow marks. At last the bond on the chlorine atom shifts towards it and it gets detached.
Finally, we have the chlorine atom detached and hydroxide gets attached to that position. This is how the halo group is replaced in aryl halides.
Note: Here apart from chlorine attached to the benzene group we have an electron withdrawing group $ - N{O_2}$ at the para position. Presence of a strong electron withdrawing group at ortho or para position enhances the reactivity of aryl halides towards nucleophilic substitution.
We can also say that with the presence of an electron withdrawing group the reaction can occur in less temperature and pressure.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




