
Write the mechanism involved in the esterification of a carboxylic acid with alcohol.
Answer
567k+ views
Hint: To solve this question we need to understand the meaning of esterification. The chemical reaction that takes place during the formation of the ester is called esterification. An ester can be obtained by the esterification reaction of an alcohol and a carboxylic acid.
Complete answer:
We can define esterification as the process which involves combination of an organic acid (RCOOH group) and an alcohol (ROH group) that leads to the formation of an ester (RCOOR group) and water. It is also said to be a chemical reaction that results in the formation of at least one ester product.
Thus, we can understand that when a primary alcohol is reacted with a carboxylic acid in the presence of sulfuric acid then a chemical compound is formed. Here, the compound which is found in the reaction has a sweet smell. Thus, the compound formed is known as ester. Now, let us take a look in the chemical reaction involved in the formation of the ester which is known as an esterification reaction.
$C{H_3}COOH + C{H_3}C{H_2}COOH \to C{H_3}COOC{H_2}C{H_3}$
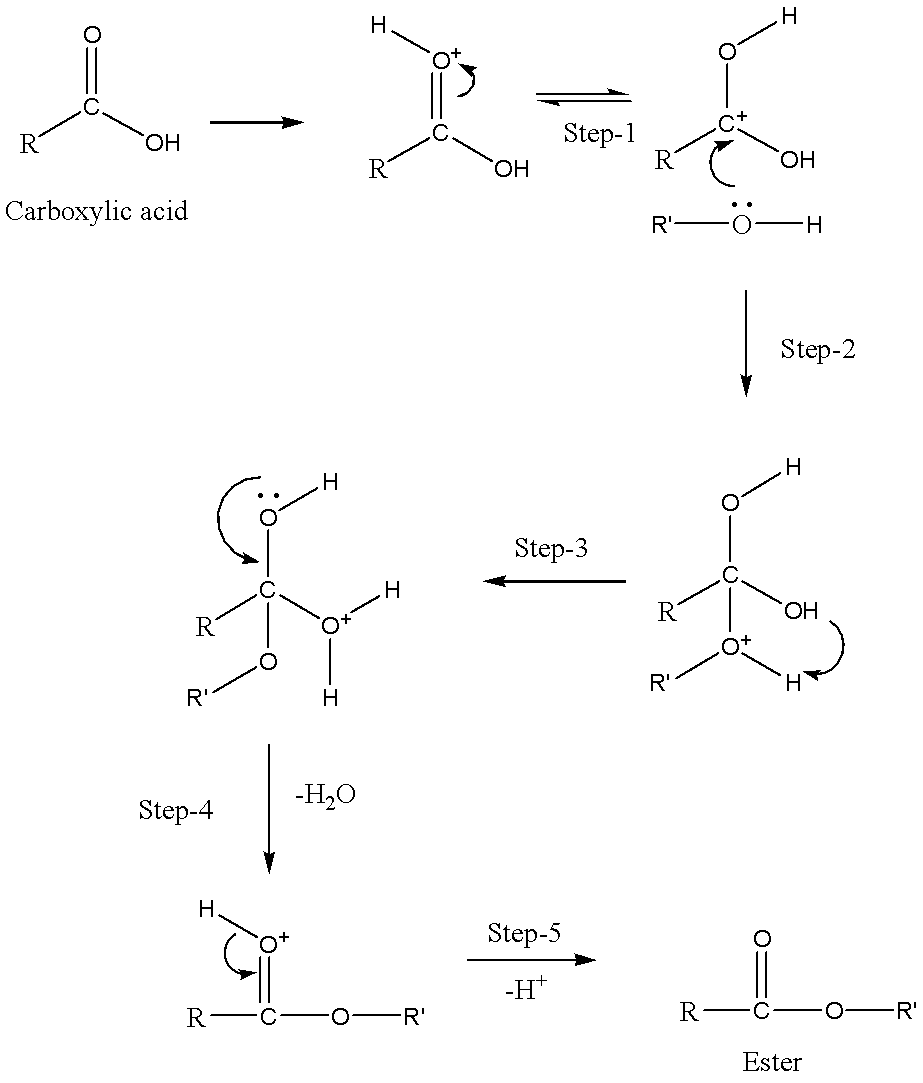
Now, let’s understand the mechanism behind esterification. The five steps involved in the process of esterification are:
Step 1: Formation of cation:
We must know that the carbocation is formed by abstracting one hydrogen.
Step 2: Delocalization of carbocation:
When the carboxyl oxygen gets protonated it gives delocalized carbocation which makes this carbocation a better electrophile.
Step 3: Transfer of proton:
After the delocalization of carbocation a proton is transferred to one of the hydroxyl groups. Thus, forms a good leaving group in the reaction.
Step 4: Removal of water molecule:
In this step, the hydroxy group’s of alcohol oxygen atom donates a pair of electrons to a carbon atom which makes a pi-bond by eliminating water. The concentration of water is less than methanol.
Therefore, it is not a feasible nucleophile to reverse this reaction.
Step 5: Formation of ester:
At last, ester formation takes place by the elimination of hydrogen.
We can write the overall mechanism as,
Note:
After esterification, we need to understand the basic properties of esterification:
-Ester is the name derived from its carboxylic acid.
-Ester has a pleasant smell.
-Used in the manufacture of perfume and food industries.
-Esters are organic compounds found in oils and fats.
Complete answer:
We can define esterification as the process which involves combination of an organic acid (RCOOH group) and an alcohol (ROH group) that leads to the formation of an ester (RCOOR group) and water. It is also said to be a chemical reaction that results in the formation of at least one ester product.
Thus, we can understand that when a primary alcohol is reacted with a carboxylic acid in the presence of sulfuric acid then a chemical compound is formed. Here, the compound which is found in the reaction has a sweet smell. Thus, the compound formed is known as ester. Now, let us take a look in the chemical reaction involved in the formation of the ester which is known as an esterification reaction.
$C{H_3}COOH + C{H_3}C{H_2}COOH \to C{H_3}COOC{H_2}C{H_3}$
Now, let’s understand the mechanism behind esterification. The five steps involved in the process of esterification are:
Step 1: Formation of cation:
We must know that the carbocation is formed by abstracting one hydrogen.
Step 2: Delocalization of carbocation:
When the carboxyl oxygen gets protonated it gives delocalized carbocation which makes this carbocation a better electrophile.
Step 3: Transfer of proton:
After the delocalization of carbocation a proton is transferred to one of the hydroxyl groups. Thus, forms a good leaving group in the reaction.
Step 4: Removal of water molecule:
In this step, the hydroxy group’s of alcohol oxygen atom donates a pair of electrons to a carbon atom which makes a pi-bond by eliminating water. The concentration of water is less than methanol.
Therefore, it is not a feasible nucleophile to reverse this reaction.
Step 5: Formation of ester:
At last, ester formation takes place by the elimination of hydrogen.
We can write the overall mechanism as,

Note:
After esterification, we need to understand the basic properties of esterification:
-Ester is the name derived from its carboxylic acid.
-Ester has a pleasant smell.
-Used in the manufacture of perfume and food industries.
-Esters are organic compounds found in oils and fats.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




