
Write the main product of the following reaction?
(I)
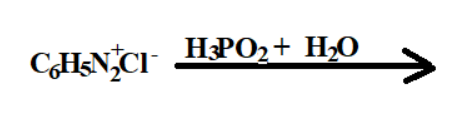
(II)
(III)
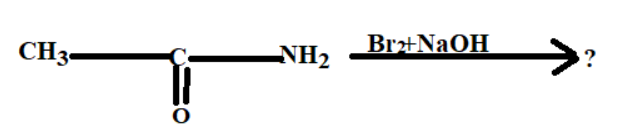
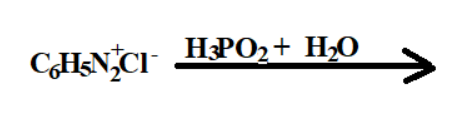

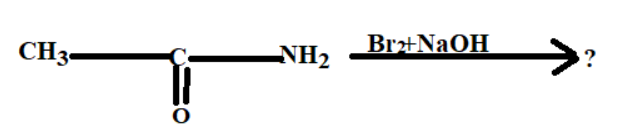
Answer
581.7k+ views
Hint: Benzene is an organic compound hence. This reaction falls under organic compound reaction. Hence bond creation and breaking in organic compound reaction should be taken in consideration while solving.
Complete step by step answer:
(I)
First of all let's see the IUPAC name and structure of the reactants
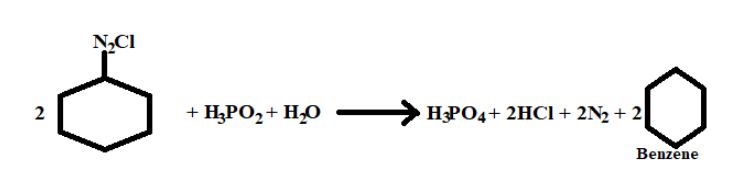
1. The first reactant is \[{C_6}{H_5}N_2^ + C{l^ - }\] it-s IUPAC name is Benzenediazonium chloride
2. The second reactant is ${H_3}P{O_2}$ and its IUPAC name is Hypo phosphorous acid
3. The third reactant is ${H_{_2}}O$ its IUPAC name is dihydrogen oxide.
Reaction :-
1. The first reactant the diazonium is a very good leaving element, its positively charged. While breaking bond with phenyl it takes away two electrons with it.
2. Now the phenyl has developed two positive charge ions and the incoming element has to be nucleophile.
3. The first incoming element that is hypophosphorous acid breaks its bond with one of the hydrogen attached with oxygen and gives one of its electrons of oxygen to the phenyl ion.
4. The water present in acidic medium breaks bond with one of its hydrogen with oxygen and gives its electron to phenyl by forming covalent bond.
5. Hence after the full reaction the final result is formation of phenol, along with formation of by products like nitrogen gas and Hcl acid.
(II) Now the IUPAC name of Reactants are
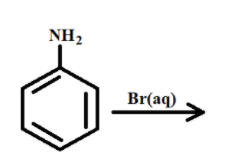
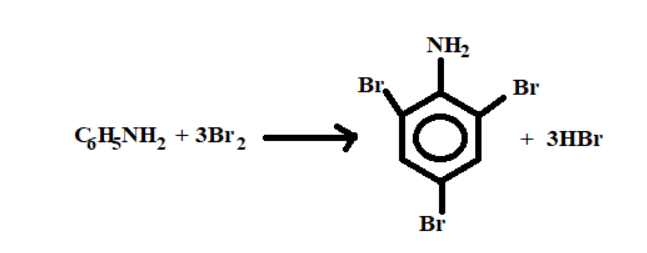
1. The first reactant is ${C_6}{H_5}N{H_2}$, its IUPAC name is Aniline
2. The second reactant is $Br_2$, its IUPAC name is Bromine.
3. The third reactant is water.
Reaction:-
1. The Aniline present in the aqueous medium breaks the hydrogen bonds and has 2 valence electrons .
2. The Bromine present in the solution forms 2 covalent bonds with the valance electrons of the phenyl.
3. The final compound formed after chemical reaction is 2,4,6-tribromoaniline and its bi-products are hydromronine.
(iii) Now the IUPAC name of Reactants are
1. The first reactant is $C{H_3}CON{H_2}$, its IUPAC name is Acetamide
2. The second reactant is $Br_2$, its IUPAC name is Bromine.
3. NaOH third reactant is sodium hydroxide.
Reaction:-
1. The Acetamide present in the aqueous medium breaks the covalent bond with carbon monoxide and forms methylamine.
2. The Bromine present in the solution forms an ionic bond with the sodium atom.
3. The final compound formed after chemical reaction is 2methylamine and its bi-products are sodium bromide and sodium carbonate.
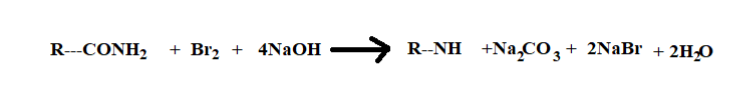
Note:
While solving the question students tend to make mistakes in bond breaking, creation, electron transfer and overall charge determination of the compound. Hence proper schematic solution to be provided with keeping in track of electron and charge transfer.
Complete step by step answer:
(I)
First of all let's see the IUPAC name and structure of the reactants
1. The first reactant is \[{C_6}{H_5}N_2^ + C{l^ - }\] it-s IUPAC name is Benzenediazonium chloride
2. The second reactant is ${H_3}P{O_2}$ and its IUPAC name is Hypo phosphorous acid
3. The third reactant is ${H_{_2}}O$ its IUPAC name is dihydrogen oxide.
Reaction :-
1. The first reactant the diazonium is a very good leaving element, its positively charged. While breaking bond with phenyl it takes away two electrons with it.
2. Now the phenyl has developed two positive charge ions and the incoming element has to be nucleophile.
3. The first incoming element that is hypophosphorous acid breaks its bond with one of the hydrogen attached with oxygen and gives one of its electrons of oxygen to the phenyl ion.
4. The water present in acidic medium breaks bond with one of its hydrogen with oxygen and gives its electron to phenyl by forming covalent bond.
5. Hence after the full reaction the final result is formation of phenol, along with formation of by products like nitrogen gas and Hcl acid.

(II) Now the IUPAC name of Reactants are
1. The first reactant is ${C_6}{H_5}N{H_2}$, its IUPAC name is Aniline
2. The second reactant is $Br_2$, its IUPAC name is Bromine.
3. The third reactant is water.
Reaction:-
1. The Aniline present in the aqueous medium breaks the hydrogen bonds and has 2 valence electrons .
2. The Bromine present in the solution forms 2 covalent bonds with the valance electrons of the phenyl.
3. The final compound formed after chemical reaction is 2,4,6-tribromoaniline and its bi-products are hydromronine.

(iii) Now the IUPAC name of Reactants are
1. The first reactant is $C{H_3}CON{H_2}$, its IUPAC name is Acetamide
2. The second reactant is $Br_2$, its IUPAC name is Bromine.
3. NaOH third reactant is sodium hydroxide.
Reaction:-
1. The Acetamide present in the aqueous medium breaks the covalent bond with carbon monoxide and forms methylamine.
2. The Bromine present in the solution forms an ionic bond with the sodium atom.
3. The final compound formed after chemical reaction is 2methylamine and its bi-products are sodium bromide and sodium carbonate.
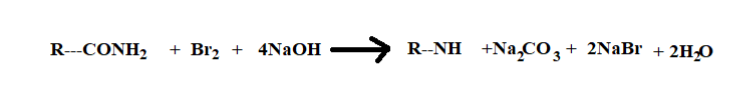
Note:
While solving the question students tend to make mistakes in bond breaking, creation, electron transfer and overall charge determination of the compound. Hence proper schematic solution to be provided with keeping in track of electron and charge transfer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




