
Write the IUPAC names of phenols given:
(i)-
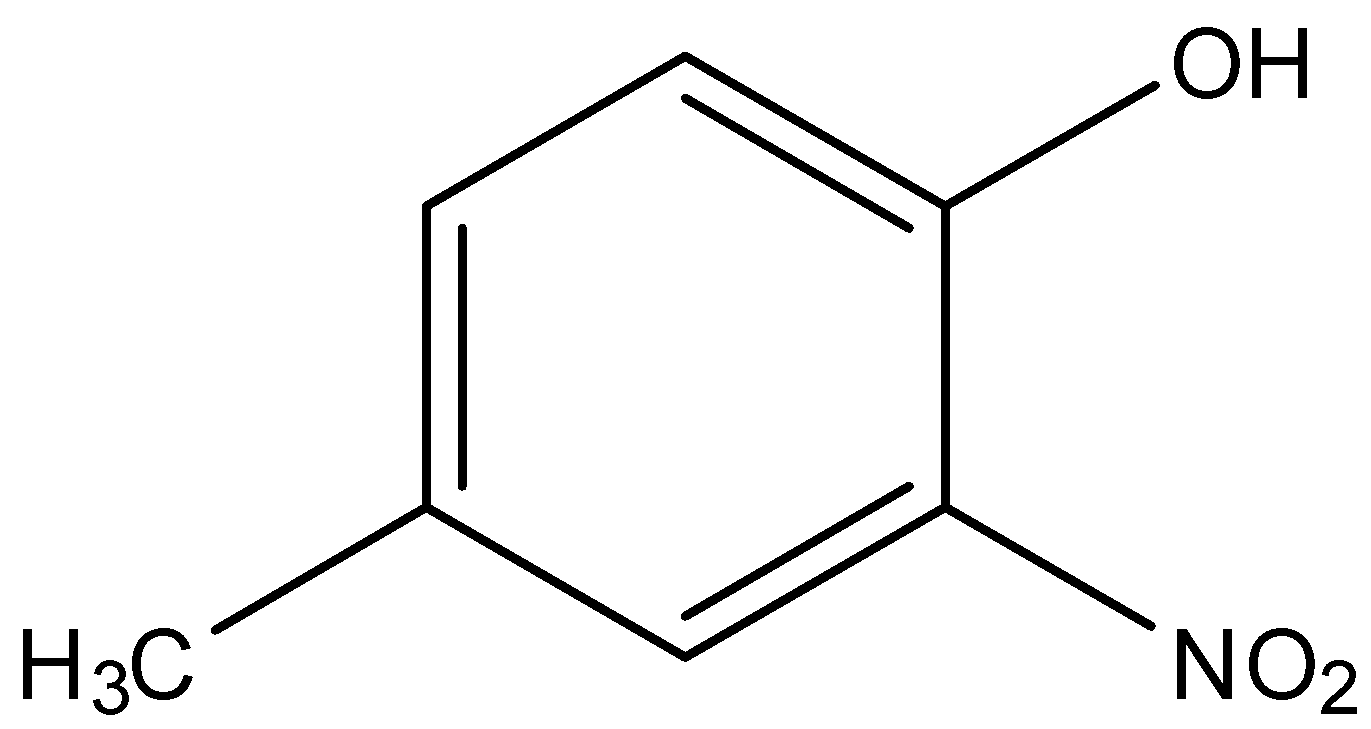
(ii)-
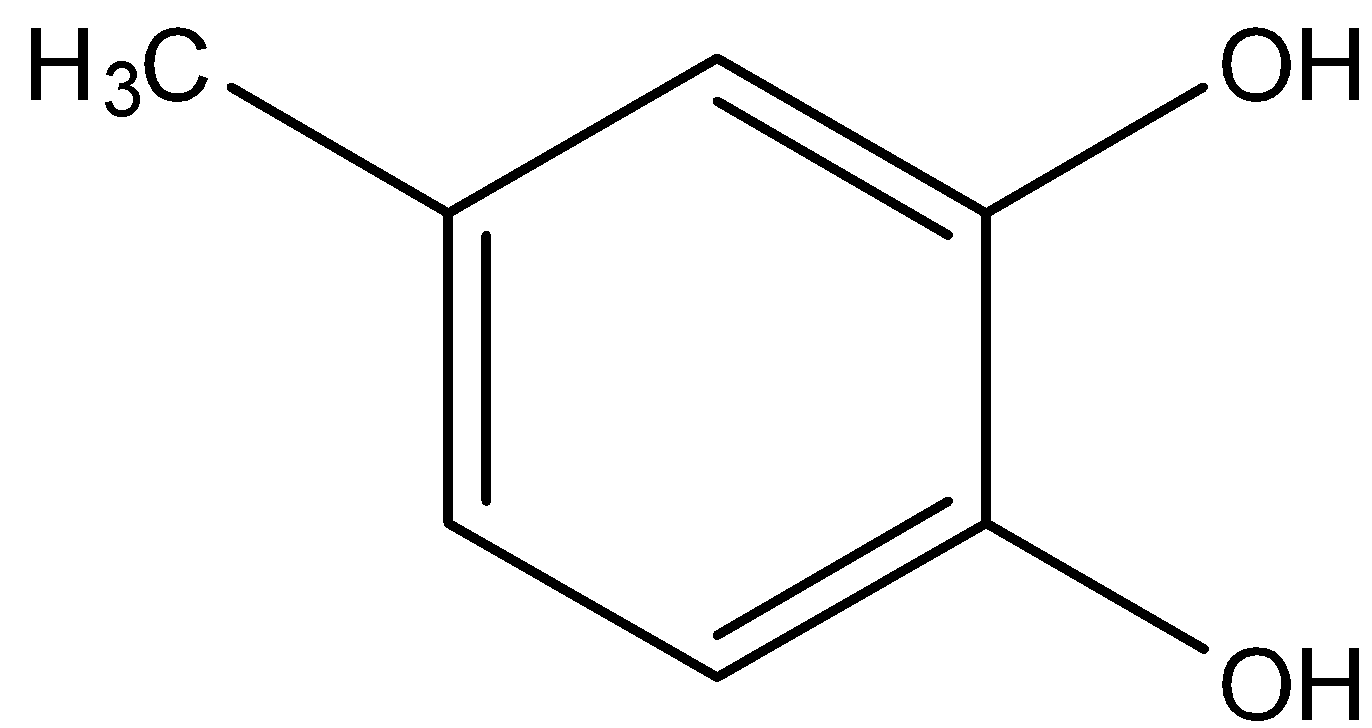


Answer
531.9k+ views
Hint: Phenols are those compounds in which there is a hydroxyl group present on the benzene ring. In option (i), the other two substituents are nitro and methyl groups, and in option (ii), there are two hydroxyl groups and one methyl group present on the benzene ring. While naming the compound, the methyl group will be named in the prefix part.
Complete answer:
There are many aromatic compounds in which phenols are those in which there is a hydroxyl group present on the benzene ring.
While naming in the IUPAC, we have to see which group should be given preference. The group that is given preference is named in the suffix part.
In option (i), there are two substituents on the phenol molecule, these are the nitro group and the methyl group.
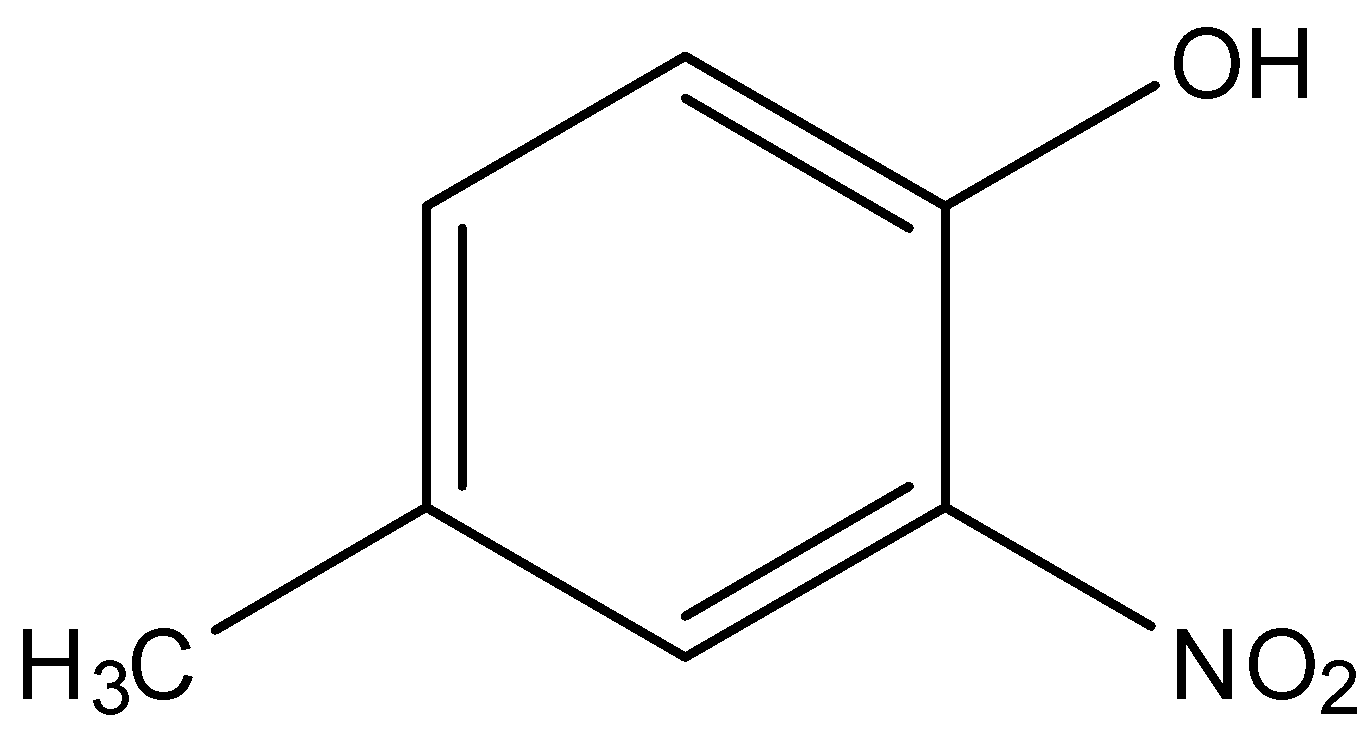
The nitro group is on the ortho or 2nd position of the phenol, while the methyl group is on the para or 4th position of the phenol. While naming the methyl group will be named first and the nitro group will be named later. So, the name will be 4-Methyl-2-nitrophenol.
In option (b), there are two hydroxyl groups and one methyl, so in this molecule, one hydroxyl group is present in the 1st position, the other hydroxyl group is in the 2nd position and the methyl group is present in the 4th position.
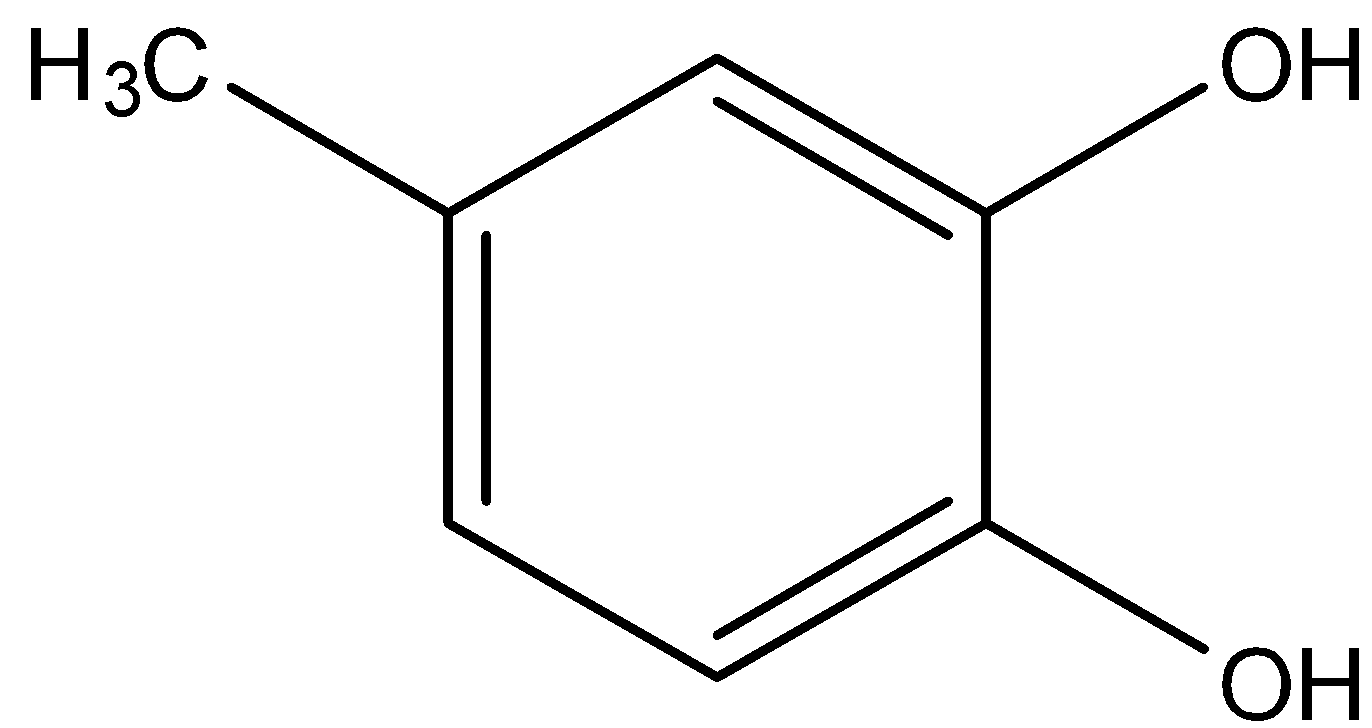
So, its name will be 4-methyl benzene-1, 2-diol.
Note:
While naming the aromatic compound, the 2nd position can also be named as ortho, the 3rd position can also be named as meta, and the 4th position can also be named as para.
Complete answer:
There are many aromatic compounds in which phenols are those in which there is a hydroxyl group present on the benzene ring.
While naming in the IUPAC, we have to see which group should be given preference. The group that is given preference is named in the suffix part.
In option (i), there are two substituents on the phenol molecule, these are the nitro group and the methyl group.

The nitro group is on the ortho or 2nd position of the phenol, while the methyl group is on the para or 4th position of the phenol. While naming the methyl group will be named first and the nitro group will be named later. So, the name will be 4-Methyl-2-nitrophenol.
In option (b), there are two hydroxyl groups and one methyl, so in this molecule, one hydroxyl group is present in the 1st position, the other hydroxyl group is in the 2nd position and the methyl group is present in the 4th position.

So, its name will be 4-methyl benzene-1, 2-diol.
Note:
While naming the aromatic compound, the 2nd position can also be named as ortho, the 3rd position can also be named as meta, and the 4th position can also be named as para.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




