
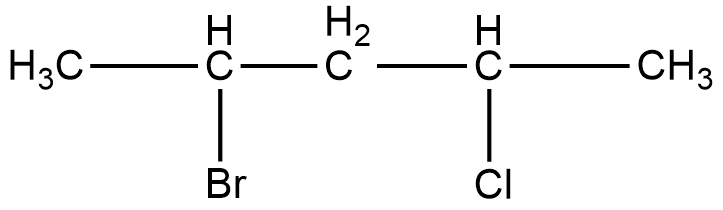
Write the IUPAC name of the following compound.

Answer
548.7k+ views
Hint: The given molecule is an organic molecule. IUPAC (International Union of Pure and Applied Chemistry) has proposed certain rules for the nomenclature of organic compounds. Following those rules, we can name all the organic molecules.
Complete step by step answer:
Given compound is an organic molecule containing both bromine and chlorine.
The steps for naming a simple organic molecule like this are,
a.Identify the longest carbon chain. It is the parental chain.
b.Count the number of carbons in that chain and name according to that. For one carbon it is methane, for two carbon it is ethane, for three carbon it is propane etc.
c.Identify side chains or functional groups if present.
d.Position of the side chain(s) or functional group(s) should be mentioned in the name. For that, numbering is done following the priority order. While numbering multiple side chains, the one with least sum should be taken.
e.If multiple side chains of the same atom or group are present, a number of side chains should be mentioned using the prefixes di-, tri-,tetra etc.
Now let us name the given molecule.
The longest carbon chain contains five carbons. Hence the parental chain is pentane. There are two functional groups present. These are bromine and chlorine. Numbering from left gives second position to Br and fourth position to Cl. Numbering from right gives fourth position to Br and second position to Cl. Br has more priority than Cl. Hence numbering is done from left. There are no other functional groups present. Hence IUPAC name of the compound will be,
\[{\text{2 - bromo - 4 - chloropentane}}\]
Note:
Before naming an organic compound, the knowledge about possible side chains and their IUPAC name is necessary. We should also know the priority order of different functional groups.
Complete step by step answer:
Given compound is an organic molecule containing both bromine and chlorine.
The steps for naming a simple organic molecule like this are,
a.Identify the longest carbon chain. It is the parental chain.
b.Count the number of carbons in that chain and name according to that. For one carbon it is methane, for two carbon it is ethane, for three carbon it is propane etc.
c.Identify side chains or functional groups if present.
d.Position of the side chain(s) or functional group(s) should be mentioned in the name. For that, numbering is done following the priority order. While numbering multiple side chains, the one with least sum should be taken.
e.If multiple side chains of the same atom or group are present, a number of side chains should be mentioned using the prefixes di-, tri-,tetra etc.
Now let us name the given molecule.
The longest carbon chain contains five carbons. Hence the parental chain is pentane. There are two functional groups present. These are bromine and chlorine. Numbering from left gives second position to Br and fourth position to Cl. Numbering from right gives fourth position to Br and second position to Cl. Br has more priority than Cl. Hence numbering is done from left. There are no other functional groups present. Hence IUPAC name of the compound will be,
\[{\text{2 - bromo - 4 - chloropentane}}\]
Note:
Before naming an organic compound, the knowledge about possible side chains and their IUPAC name is necessary. We should also know the priority order of different functional groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




