
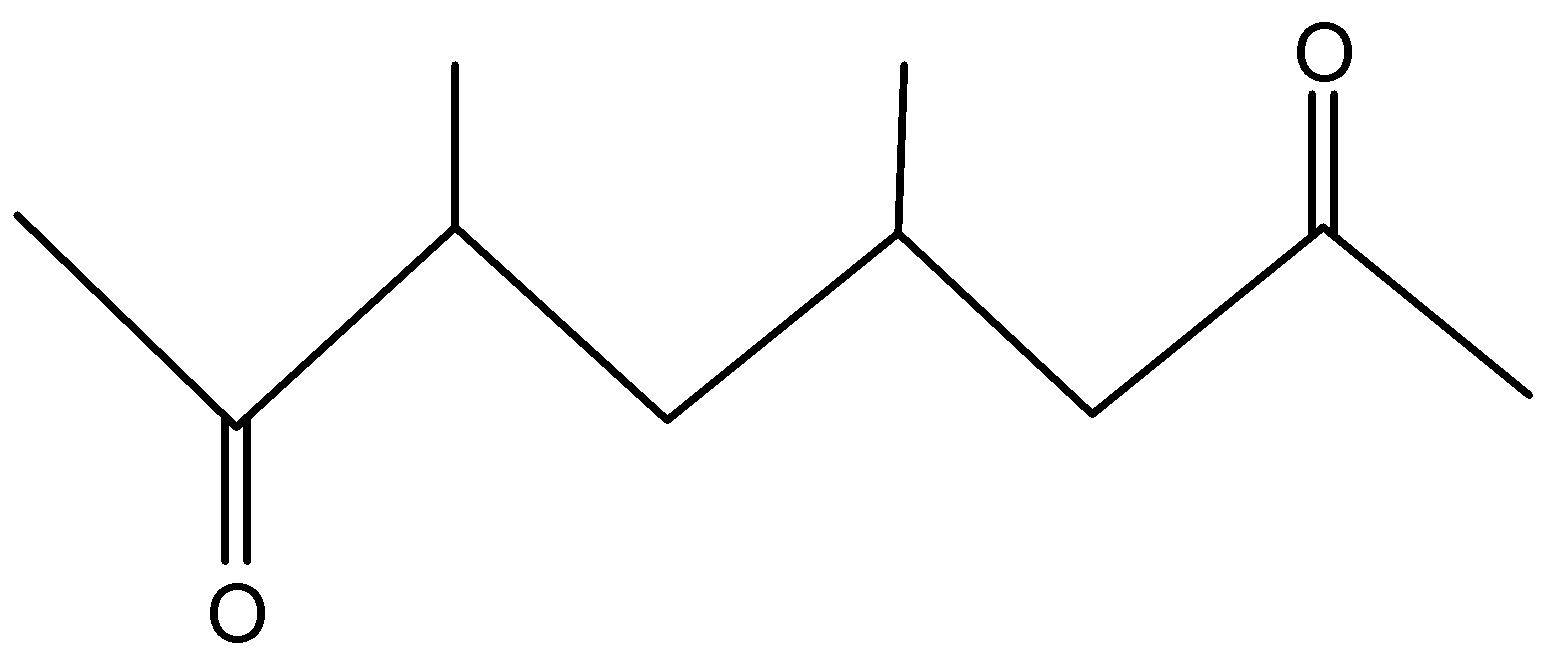
Write the IUPAC name of the following –

Answer
568.2k+ views
Hint: While naming an organic compound by IUPAC rules, we first find out the longest continuous chain of C atoms i.e. parent chain.
Complete Solution:
First we will find out the parent chain and the number of C atoms in it. We will number the chain in such a way that the C atom near the functional group gets the lowest number. In this case, even if we begin from either side, it will be the same.
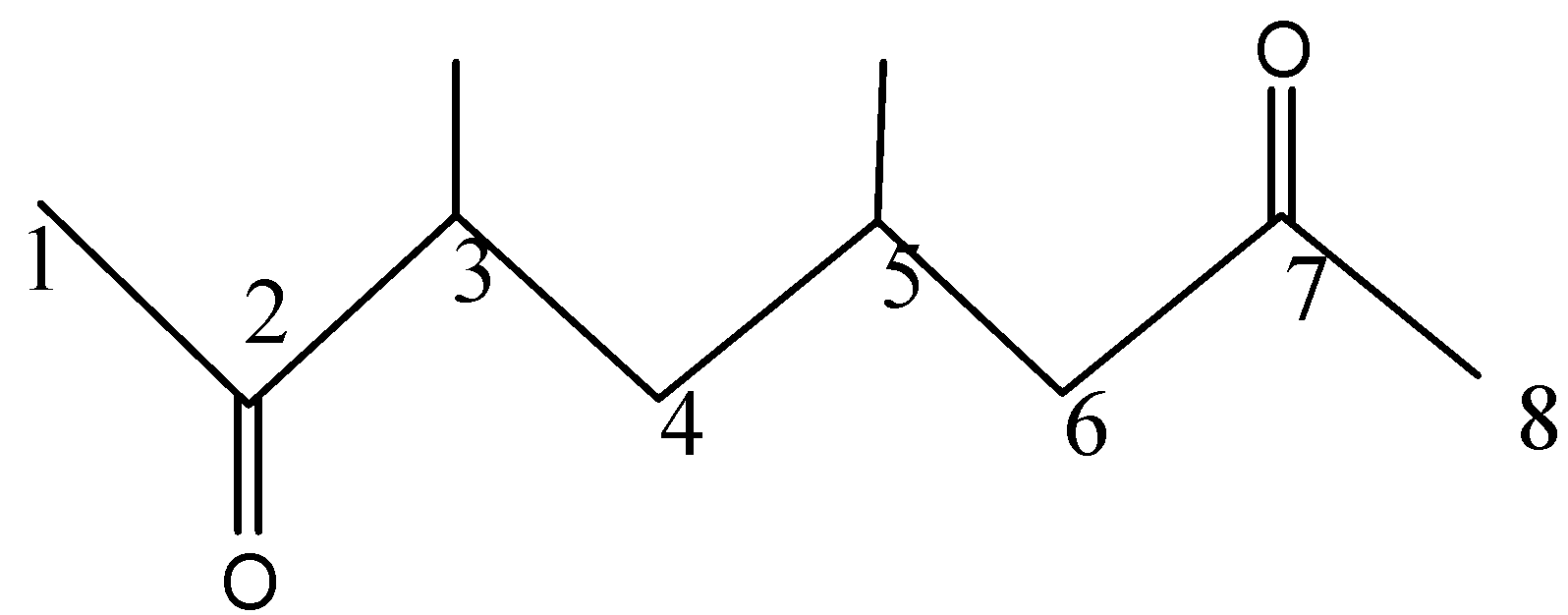
Parent compound is Octane.
- Now, as per IUPAC rules, the ketonic group is given high priority and hence is considered as a parent chain. Also, the doubly bonded oxygen is present at 2 positions so we will add the prefix “di” i.e. dione.
So the parent chain is octan-2,7-dione.
- At, 3rd and 5th Carbon a methyl group is attached. So that will be written as ‘3,5 -dimethyl’ which is our substituent.
- The format of IUPAC name of an organic compound is Locant + prefix + root + locant + suffix.
According to which the correct IUPAC name of given compound becomes,
3,5-dimethyloctan-2,7-dione.
Additional information: IUPAC is a systematic approach for nomenclature of organic compounds as per the rules set by International Union of Pure and Applied Chemistry.
While naming compounds in the IUPAC system, all the compounds containing C as the principal element are considered as ‘organic compounds’. Other elements such as O, H and N which are usually associated with C are considered as functional groups.
Note: We have to remember that the ketonic group is given higher priority than alkane. When we count the parent chain, always consider all possible combinations in order to get the highest number of C atoms in the parent chain.
Complete Solution:
First we will find out the parent chain and the number of C atoms in it. We will number the chain in such a way that the C atom near the functional group gets the lowest number. In this case, even if we begin from either side, it will be the same.

Parent compound is Octane.
- Now, as per IUPAC rules, the ketonic group is given high priority and hence is considered as a parent chain. Also, the doubly bonded oxygen is present at 2 positions so we will add the prefix “di” i.e. dione.
So the parent chain is octan-2,7-dione.
- At, 3rd and 5th Carbon a methyl group is attached. So that will be written as ‘3,5 -dimethyl’ which is our substituent.
- The format of IUPAC name of an organic compound is Locant + prefix + root + locant + suffix.
According to which the correct IUPAC name of given compound becomes,
3,5-dimethyloctan-2,7-dione.
Additional information: IUPAC is a systematic approach for nomenclature of organic compounds as per the rules set by International Union of Pure and Applied Chemistry.
While naming compounds in the IUPAC system, all the compounds containing C as the principal element are considered as ‘organic compounds’. Other elements such as O, H and N which are usually associated with C are considered as functional groups.
Note: We have to remember that the ketonic group is given higher priority than alkane. When we count the parent chain, always consider all possible combinations in order to get the highest number of C atoms in the parent chain.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




