
Write the I.U.P.A.C name of following compound
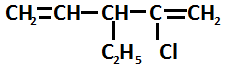
Answer
510k+ views
Hint: For the purpose of rationalizing the system of naming of different chemical structures, an international congress of chemists adopted the universally accepted system for naming the chemical structure known as the I.U.P.A.C system.
Complete answer:
To write the IUPAC for a given structure we must follow some basic steps.
IUPAC of compound is represented as
PREFIX + WORD ROOT + PRIMARY SUFFIX+ SECONDARY SUFFIX
Select the word root which contains the maximum number of carbons in a single chain. Following this rule, we find that naming the terminal carbon atom as 1 and finally we get a carbon chain of 5 atoms.
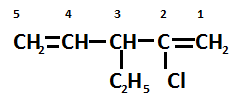
As we noticed from the structure there are two double bonds present. So, add the suffix $\left( { - ene} \right)$ which indicates the presence of a double bond in the structure.
Numerical suffix $\left( { - di} \right)$ must be added before the $\left( { - ene} \right)$ which indicate the total number of double bonds present in a molecule.
Use a numerical digit $\left( {1,5} \right)$ to show the position of double bond in the molecule.
Halogen atoms (chlorine) $\left( {Cl} \right)$ act as secondary suffixes in the molecule.
Numbering should be marked starting from the carbon atom from which chlorine atom is present at lowest number.
Following this rule, we give the number $\left( 2 \right)$ to the halogen atom.
Ethane group $\left( {{C_2}{H_5}} \right)$ present in the molecule do not come in the primary word root and hence, act as substituent or commonly known as Prefix.
When we consider ethane as a substituent it is named as ethyl $\left( { - {C_2}{H_5}} \right)$ which is present at position $\left( 3 \right)$.
Name of the substituent is arranged alphabetically hence, ethyl comes first than chloro.
Finally, the IUPAC of the compound is $2 - $chloro,$3 - $ethyl-pent $1,4$$ - diene$.
Note:
Remember to select the longest carbon chain in the starting of writing the IUPAC name.
When we add any primary suffix with a secondary suffix, always remember to eliminate the terminal (e) alphabet from the name of the primary suffix.
If more than one functional group is present, then numbering is done depending upon their priority.
Complete answer:
To write the IUPAC for a given structure we must follow some basic steps.
IUPAC of compound is represented as
PREFIX + WORD ROOT + PRIMARY SUFFIX+ SECONDARY SUFFIX
Select the word root which contains the maximum number of carbons in a single chain. Following this rule, we find that naming the terminal carbon atom as 1 and finally we get a carbon chain of 5 atoms.
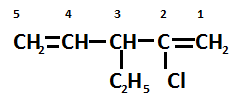
As we noticed from the structure there are two double bonds present. So, add the suffix $\left( { - ene} \right)$ which indicates the presence of a double bond in the structure.
Numerical suffix $\left( { - di} \right)$ must be added before the $\left( { - ene} \right)$ which indicate the total number of double bonds present in a molecule.
Use a numerical digit $\left( {1,5} \right)$ to show the position of double bond in the molecule.
Halogen atoms (chlorine) $\left( {Cl} \right)$ act as secondary suffixes in the molecule.
Numbering should be marked starting from the carbon atom from which chlorine atom is present at lowest number.
Following this rule, we give the number $\left( 2 \right)$ to the halogen atom.
Ethane group $\left( {{C_2}{H_5}} \right)$ present in the molecule do not come in the primary word root and hence, act as substituent or commonly known as Prefix.
When we consider ethane as a substituent it is named as ethyl $\left( { - {C_2}{H_5}} \right)$ which is present at position $\left( 3 \right)$.
Name of the substituent is arranged alphabetically hence, ethyl comes first than chloro.
Finally, the IUPAC of the compound is $2 - $chloro,$3 - $ethyl-pent $1,4$$ - diene$.
Note:
Remember to select the longest carbon chain in the starting of writing the IUPAC name.
When we add any primary suffix with a secondary suffix, always remember to eliminate the terminal (e) alphabet from the name of the primary suffix.
If more than one functional group is present, then numbering is done depending upon their priority.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




