
Write the IUPAC name of $ (CH_3)_3CCHO $ ?
Answer
507.6k+ views
Hint :The International Union of Pure and Applied Chemistry (IUPAC) has given rules for naming of both organic and inorganic compounds called the IUPAC system of nomenclature. However any chemical compound is often given a common or trivial name which can sometimes be more common than IUPAC name of compound. There are certain rules that need to be followed in the IUPAC system of nomenclature.
Complete Step By Step Answer:
Before answering the above question we must know certain rules for naming of compounds-
1. In the compound we have 3 parts-
(a)Prefix (primary-branches or substituents, secondary-cyclo spiro or bicyclo)
(b)Word root (represents number of carbon in main chain)
Example-1C (Meth), 2C (Eth), 3C (prop), 4C (But) and so on.
(c)Suffix (primary-type of carbon bond, secondary-functional group)
The sequence is as-secondary prefix+primary prefix+word root+primary suffix +secondary suffix.
2. Select the longest carbon chain (need not to be straight).If 2 or more long carbon-carbon chains select the one with maximum number of branches. (The longest chain rule)
3. Numbering from that side in which branch gets the lowest locant (number). (The lowest set of locant)
4. The naming of substituents as per the alphabetical order.
5. If multiple same substituents are present then use the prefix-di, tri, tetra or penta etc.
6. If there are different substituents present at the same position then name is given alphabetically.
7. If 2 or more functional groups are present than naming as per the priority –
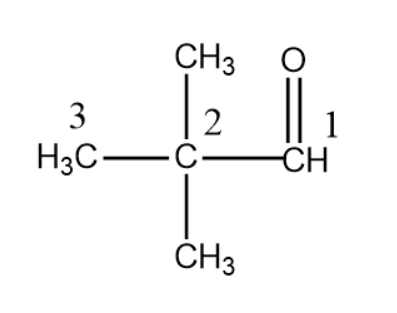
Numbering is started from the Aldehyde group and we obtain a 3 membered parent chain so we use prop- ,2 methyl substituents at 2nd place so dimethyl will be used and since aldehyde is the only functional group we use suffix –al.
So the name is 2, 2-dimethyl propan-1-al.
Note :
One thing is to be noted that we have removed “e” after the propane. This is because when a, e, i, o, u or y are repeated one of them is removed. When more than 1 functional group is present and the aldehyde functional group is not the primary functional group we use “oxo” as a prefix when the $ - CHO $ group is counted in the chain but when not counted we use the prefix “formyl”.
Complete Step By Step Answer:
Before answering the above question we must know certain rules for naming of compounds-
1. In the compound we have 3 parts-
(a)Prefix (primary-branches or substituents, secondary-cyclo spiro or bicyclo)
(b)Word root (represents number of carbon in main chain)
Example-1C (Meth), 2C (Eth), 3C (prop), 4C (But) and so on.
(c)Suffix (primary-type of carbon bond, secondary-functional group)
The sequence is as-secondary prefix+primary prefix+word root+primary suffix +secondary suffix.
2. Select the longest carbon chain (need not to be straight).If 2 or more long carbon-carbon chains select the one with maximum number of branches. (The longest chain rule)
3. Numbering from that side in which branch gets the lowest locant (number). (The lowest set of locant)
4. The naming of substituents as per the alphabetical order.
5. If multiple same substituents are present then use the prefix-di, tri, tetra or penta etc.
6. If there are different substituents present at the same position then name is given alphabetically.
7. If 2 or more functional groups are present than naming as per the priority –
| Group | Suffix | Prefix |
| $ 1. - COOH $ | oic acid | carboxy |
| $ 2. - S{O_3}H $ | sulphonic acid | sulpho |
| $ 3. - COOCO - $ | oic anhydride | ----- |
| $ 4. - RCOO{R^'} $ | Alkyl alkanoate | alkoxy carbonyl |
| $ 5. - COX $ | oyl halide | halo formyl |
| $ 6. - CON{H_2} $ | amide | carbamoyl |
| $ 7. - CN $ | Nitrile | cyano |
| $ 8. - NC $ | isonitrile | isocyano |
| $ 9. - CHO $ | al | formyl or oxo |
| $ 10. - CO - $ | one | oxo |
| $ 11. - OH $ | ol | hydroxy |
| $ 12. - SH $ | thiol | mercapto |
| $ 13. - N{H_2} $ | amine | amino |

Numbering is started from the Aldehyde group and we obtain a 3 membered parent chain so we use prop- ,2 methyl substituents at 2nd place so dimethyl will be used and since aldehyde is the only functional group we use suffix –al.
So the name is 2, 2-dimethyl propan-1-al.
Note :
One thing is to be noted that we have removed “e” after the propane. This is because when a, e, i, o, u or y are repeated one of them is removed. When more than 1 functional group is present and the aldehyde functional group is not the primary functional group we use “oxo” as a prefix when the $ - CHO $ group is counted in the chain but when not counted we use the prefix “formyl”.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




