
Write the isomers of the compound having formula \[{C_4}{H_9}Br\] .
Answer
469.2k+ views
Hint: The isomers of any organic compound are the compounds having the same molecular formula yet different bond linkages. The number of atoms of each element remains the same but their positions can be changed to obtain different isomers.
Complete step by step answer:
The given compound contains four carbon atoms and a bromine substituent with the formula \[{C_4}{H_9}Br\] and is therefore named butyl bromide or bromobutane.
The molecular formulas of the organic compounds like alkyl halides give us the information about the type of alkyl chain and the nature of the halogen being used a substituent but fail to tell us the position of the substituent and whether or not the carbon chain is branched.
The simple straight chain butane has two different positions, as the first and fourth position and the second and third position are equivalent in nature. Thus, bromine can be attached to the first or the second position of the straight chain butane to give \[1 - bromobu{\text{tane}}\] and \[2 - bromobu{\text{tane}}\] respectively.
The butane chain can also get branched to form \[{\text{2 - methylpropane}}\] which also has two different positions. The primary carbons and the tertiary carbon position. Thus, bromine can be attached to either of these positions to give \[{\text{1 - bromo - 2 - methylpropane}}\] and \[{\text{2 - bromo - 2 - methylpropane}}\] respectively.
\[ \Rightarrow \] Therefore, \[{C_4}{H_9}Br\] have four structural isomers that can be written as follows:

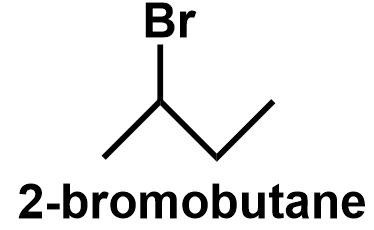
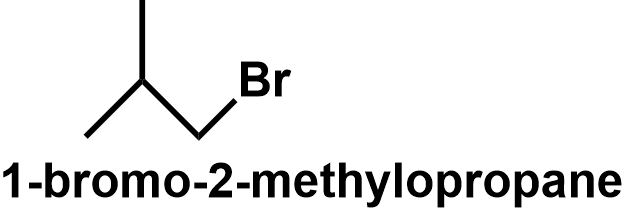
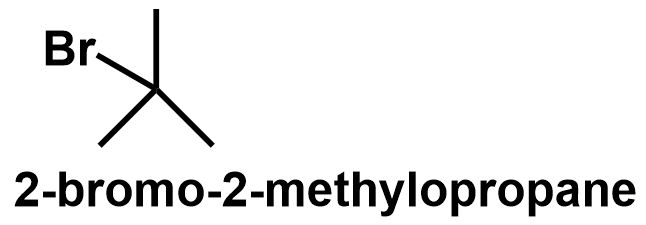
Note:
The formula does not change with the positions of bromine atoms; a bromine replaces a hydrogen in the parent chain and the number of hydrogens remain unchanged irrespective of which one is being replaced. The isomers are limited to four due to inability of the butane chain to branch further.
Complete step by step answer:
The given compound contains four carbon atoms and a bromine substituent with the formula \[{C_4}{H_9}Br\] and is therefore named butyl bromide or bromobutane.
The molecular formulas of the organic compounds like alkyl halides give us the information about the type of alkyl chain and the nature of the halogen being used a substituent but fail to tell us the position of the substituent and whether or not the carbon chain is branched.
The simple straight chain butane has two different positions, as the first and fourth position and the second and third position are equivalent in nature. Thus, bromine can be attached to the first or the second position of the straight chain butane to give \[1 - bromobu{\text{tane}}\] and \[2 - bromobu{\text{tane}}\] respectively.
The butane chain can also get branched to form \[{\text{2 - methylpropane}}\] which also has two different positions. The primary carbons and the tertiary carbon position. Thus, bromine can be attached to either of these positions to give \[{\text{1 - bromo - 2 - methylpropane}}\] and \[{\text{2 - bromo - 2 - methylpropane}}\] respectively.
\[ \Rightarrow \] Therefore, \[{C_4}{H_9}Br\] have four structural isomers that can be written as follows:



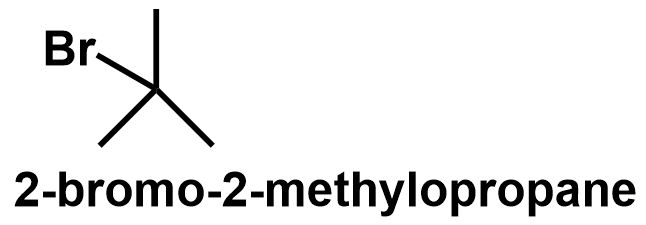
Note:
The formula does not change with the positions of bromine atoms; a bromine replaces a hydrogen in the parent chain and the number of hydrogens remain unchanged irrespective of which one is being replaced. The isomers are limited to four due to inability of the butane chain to branch further.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




