
Write the important considerations which are to be taken into account while fabricating a p-n junction diode to be used as a light emitting diode (LED). What should be the order of band gap of an LED, if it is required to emit light, in the visible range? Draw a circuit diagram and explain its action.
Answer
579k+ views
Hint: Just keep in mind that a light-emitting diode converts electrical energy into light energy. Also, A Light-emitting diode (LED) emits monochromatic light as well as white light. We can also say that light-emitting diode (LED) is a semiconductor that will glow when a voltage is applied to it.
Complete step by step solution:
Firstly, we will know about the light-emitting diode (LED).
A Light-emitting diode (LED) is defined as the special heavily doped p-n junction diode which emits spontaneous radiation when forward biased.
Now, if we want to fabricate a p-n junction, the important considerations to be taken are given as:
i) It should be a heavily doped p-n junction.
ii) The reverse breakdown of LEDs should be very low.
ii) The reverse breakdown of LED should be typically around $SV$.
Now, the semiconductor used for the LED to emit light in the visible range should have a bandgap of about $3eV$ to $1.8eV$.
Now, the circuit diagram for the light-emitting diode is given by
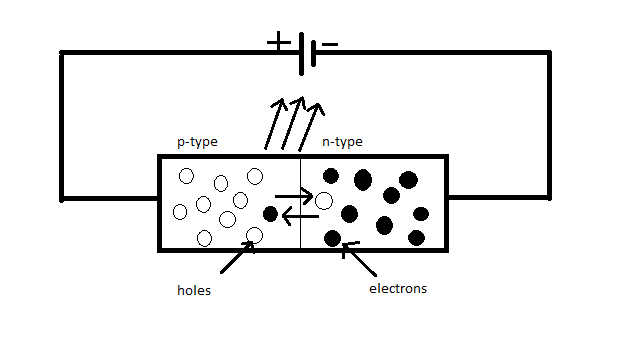
Now, we know that when a p-n junction is forward biased the electrons-injected to the p-side of the junction diode falls from the conduction band to the valence band and they will combine with the holes of the valence band. This process is equivalent to the jumping of electrons from the higher energy state to a lower energy state. Here, the energy will be released when the electron-hole recombines in the form of visible light.
Additional Information:
As we know, the energy of a photon of visible light is given by $h\nu = {E_g}$, where ${E_g}$ is the energy gap between the conduction band and valence band and $\nu $ is the frequency of emitted visible radiation. Now, the wavelength of visible light is given by
${E_g} = \dfrac{{hc}}{\lambda }$
$ \Rightarrow \,\lambda = \dfrac{{hc}}{{{E_g}}}$
$ \Rightarrow \,{E_g} \simeq \dfrac{{1.242}}{\lambda }eV$
Therefore, this is the expression. By knowing the value of energy released we can calculate the wavelength and vice-versa.
Note: Now, just remember that the V-I characteristics of LED will be the same as that of elemental semiconductor say silicon diode. Also, the knee voltage of the LED is much higher than that of the $Si$diode. But, LED’s get damaged at low reverse voltage, say $5V$.
Complete step by step solution:
Firstly, we will know about the light-emitting diode (LED).
A Light-emitting diode (LED) is defined as the special heavily doped p-n junction diode which emits spontaneous radiation when forward biased.
Now, if we want to fabricate a p-n junction, the important considerations to be taken are given as:
i) It should be a heavily doped p-n junction.
ii) The reverse breakdown of LEDs should be very low.
ii) The reverse breakdown of LED should be typically around $SV$.
Now, the semiconductor used for the LED to emit light in the visible range should have a bandgap of about $3eV$ to $1.8eV$.
Now, the circuit diagram for the light-emitting diode is given by

Now, we know that when a p-n junction is forward biased the electrons-injected to the p-side of the junction diode falls from the conduction band to the valence band and they will combine with the holes of the valence band. This process is equivalent to the jumping of electrons from the higher energy state to a lower energy state. Here, the energy will be released when the electron-hole recombines in the form of visible light.
Additional Information:
As we know, the energy of a photon of visible light is given by $h\nu = {E_g}$, where ${E_g}$ is the energy gap between the conduction band and valence band and $\nu $ is the frequency of emitted visible radiation. Now, the wavelength of visible light is given by
${E_g} = \dfrac{{hc}}{\lambda }$
$ \Rightarrow \,\lambda = \dfrac{{hc}}{{{E_g}}}$
$ \Rightarrow \,{E_g} \simeq \dfrac{{1.242}}{\lambda }eV$
Therefore, this is the expression. By knowing the value of energy released we can calculate the wavelength and vice-versa.
Note: Now, just remember that the V-I characteristics of LED will be the same as that of elemental semiconductor say silicon diode. Also, the knee voltage of the LED is much higher than that of the $Si$diode. But, LED’s get damaged at low reverse voltage, say $5V$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




