
Write the formula of the product formed when formaldehyde reacts with ammonia and name the product?
Answer
591k+ views
Hint: The formaldehyde is a group of alcohol which when reacts with ammonia then it forms a product which is usually famous for its antiseptic property and it can be used to treat urinary tract infections.
Complete step by step answer:
-In the given question we have to find the product when formaldehyde and ammonia react with each other.
-As we know that the molecular formula of formaldehyde and ammonia is $\text{HCHO and N}{{\text{H}}_{3}}$.
-So, when they both react with each other they form a cage-like structure in which the nitrogen atoms are placed at the corners and methyl groups are placed at the edges of the cage
-The balanced chemical reaction will be:
$\text{6HCHO + 4N}{{\text{H}}_{3}}\text{ }\to \text{ }{{\left( \text{C}{{\text{H}}_{2}} \right)}_{6}}{{\text{N}}_{4}}\text{ + 6}{{\text{H}}_{2}}\text{O}$
-Here, the structure of ${{\left( \text{C}{{\text{H}}_{2}} \right)}_{6}}{{\text{N}}_{4}}$ will be cage-like as shown below:
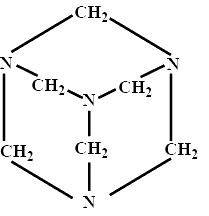
-The name of the compound is hexamethylenetetramine because as we can see that a total of 6 methyl groups are present at the edge and 4 amine groups are present at the corners of the cage-like structure.
-Along with it, a water molecule is also released at the end of the reaction.
-Also, the product formed that is hexamethylenetetramine is also known as urotropine.
-Urotropine has an antiseptic property that's why it is used to treat urinary tract infections.
-Because urotropine can decompose at the acidic pH into formaldehyde and ammonia and we know that the formaldehyde has the bactericidal property which can stop the growth of the bacteria.
Therefore, the product formed is Hexamethylenetetramine or urotropine.
Note: In the cage structure, there is no space for the bond formation with other atoms. The compound is formed on the industrial scale and it was first discovered by Aleksandr Butlerov. The compound is also highly soluble in the water.
Complete step by step answer:
-In the given question we have to find the product when formaldehyde and ammonia react with each other.
-As we know that the molecular formula of formaldehyde and ammonia is $\text{HCHO and N}{{\text{H}}_{3}}$.
-So, when they both react with each other they form a cage-like structure in which the nitrogen atoms are placed at the corners and methyl groups are placed at the edges of the cage
-The balanced chemical reaction will be:
$\text{6HCHO + 4N}{{\text{H}}_{3}}\text{ }\to \text{ }{{\left( \text{C}{{\text{H}}_{2}} \right)}_{6}}{{\text{N}}_{4}}\text{ + 6}{{\text{H}}_{2}}\text{O}$
-Here, the structure of ${{\left( \text{C}{{\text{H}}_{2}} \right)}_{6}}{{\text{N}}_{4}}$ will be cage-like as shown below:

-The name of the compound is hexamethylenetetramine because as we can see that a total of 6 methyl groups are present at the edge and 4 amine groups are present at the corners of the cage-like structure.
-Along with it, a water molecule is also released at the end of the reaction.
-Also, the product formed that is hexamethylenetetramine is also known as urotropine.
-Urotropine has an antiseptic property that's why it is used to treat urinary tract infections.
-Because urotropine can decompose at the acidic pH into formaldehyde and ammonia and we know that the formaldehyde has the bactericidal property which can stop the growth of the bacteria.
Therefore, the product formed is Hexamethylenetetramine or urotropine.
Note: In the cage structure, there is no space for the bond formation with other atoms. The compound is formed on the industrial scale and it was first discovered by Aleksandr Butlerov. The compound is also highly soluble in the water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




