
Write the electron dot structure of aluminum oxide, lithium chloride and calcium oxide?
Answer
576.3k+ views
Hint: We can say that electron dot structure is nothing but a representation of Lewis dot diagram of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots indicates the number of outermost electrons present in the atom.
Example:
The valence electron of Mg is two it can be represented in electron dot structure as,
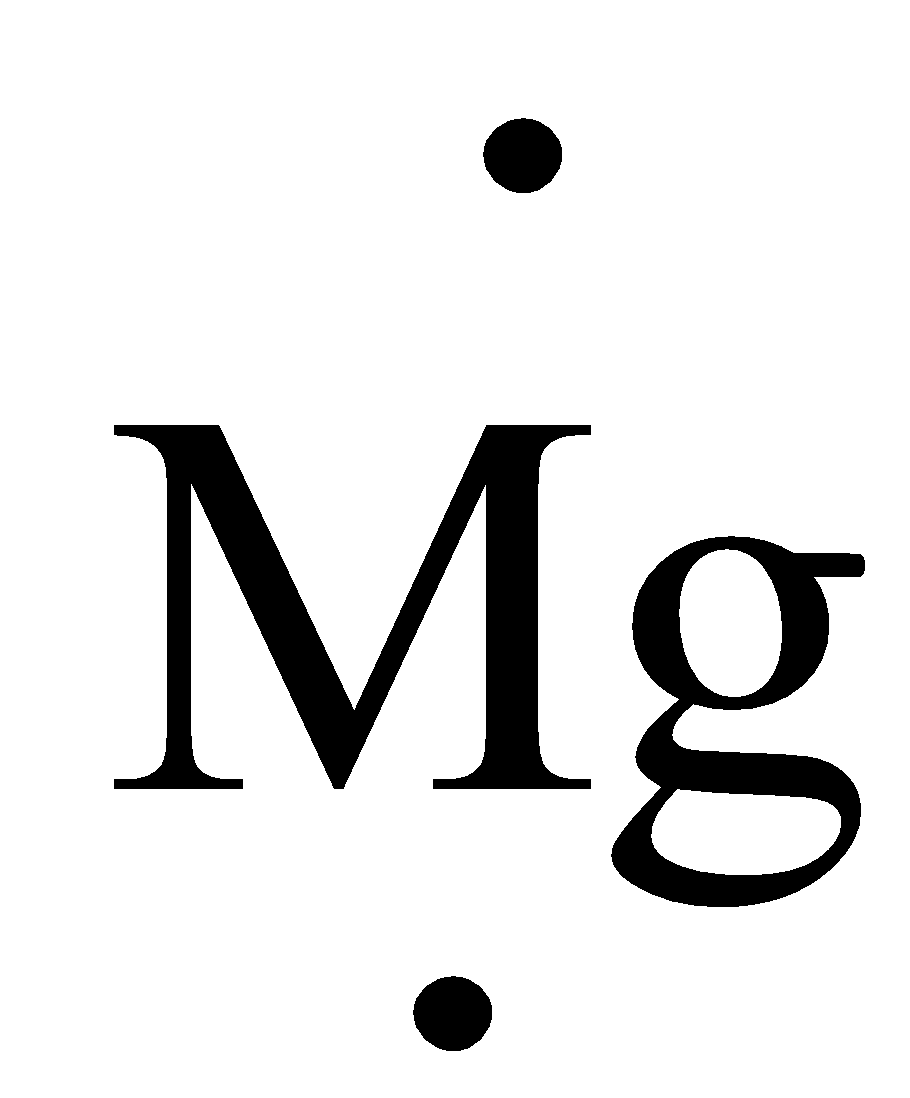
Complete step by step answer:
We can discuss about the Aluminum oxide as,
Aluminum has three valence electrons, oxygen has six valence electrons. The formula of aluminum oxide is$A{l_2}{O_3}$. The total number of valence electrons in aluminum is six and the total number of valence electrons in oxygen is eighteen. Therefore, the number of valence electrons in aluminum oxide is twenty-four. We can draw the electron dot structure of aluminum oxide as,

Let we discuss about the Lithium chloride as,
Lithium has one valence electron, chlorine has seven valence electrons. The formula of lithium chloride is $LiCl$. Therefore, the total number of valence electrons in lithium chloride is eight. We can draw the electron dot structure of lithium chloride as,

We can discuss about the Calcium oxide as,
Calcium has two valence electrons, oxygen has six valence electrons. The formula of calcium oxide is $CaO$. Therefore, the number of valence electrons in calcium oxide is eight. We can draw the electron dot structure of calcium oxide as,
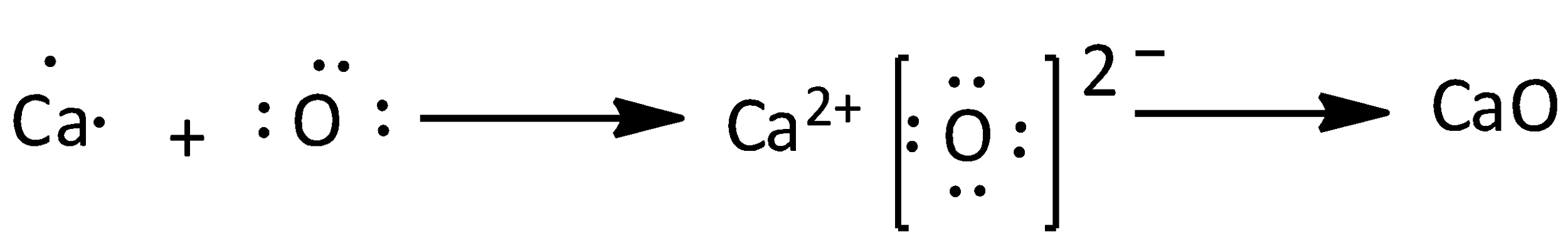
Note: We can use Lewis structure to determine the molecules geometry since it helps us to identify the outermost electrons. We could predict the geometry of a compound by,
Sketching its Lewis structure.
Calculating the number of electron pairs.
Arranging the electron pairs to decrease repulsion.
Arranging the atoms to decrease the repulsion of lone pair-lone pairs.
Naming the molecular geometries from the positions of the atom.
Example:
The valence electron of Mg is two it can be represented in electron dot structure as,
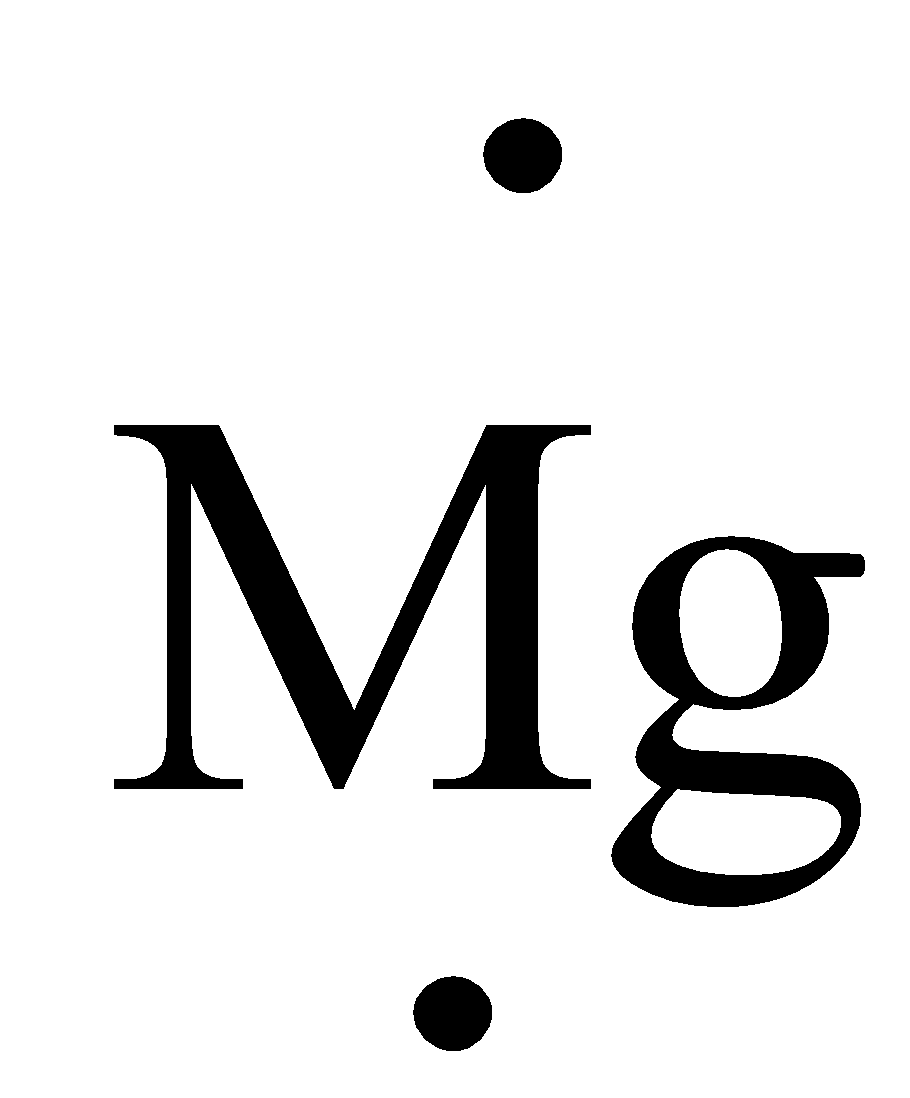
Complete step by step answer:
We can discuss about the Aluminum oxide as,
Aluminum has three valence electrons, oxygen has six valence electrons. The formula of aluminum oxide is$A{l_2}{O_3}$. The total number of valence electrons in aluminum is six and the total number of valence electrons in oxygen is eighteen. Therefore, the number of valence electrons in aluminum oxide is twenty-four. We can draw the electron dot structure of aluminum oxide as,

Let we discuss about the Lithium chloride as,
Lithium has one valence electron, chlorine has seven valence electrons. The formula of lithium chloride is $LiCl$. Therefore, the total number of valence electrons in lithium chloride is eight. We can draw the electron dot structure of lithium chloride as,

We can discuss about the Calcium oxide as,
Calcium has two valence electrons, oxygen has six valence electrons. The formula of calcium oxide is $CaO$. Therefore, the number of valence electrons in calcium oxide is eight. We can draw the electron dot structure of calcium oxide as,
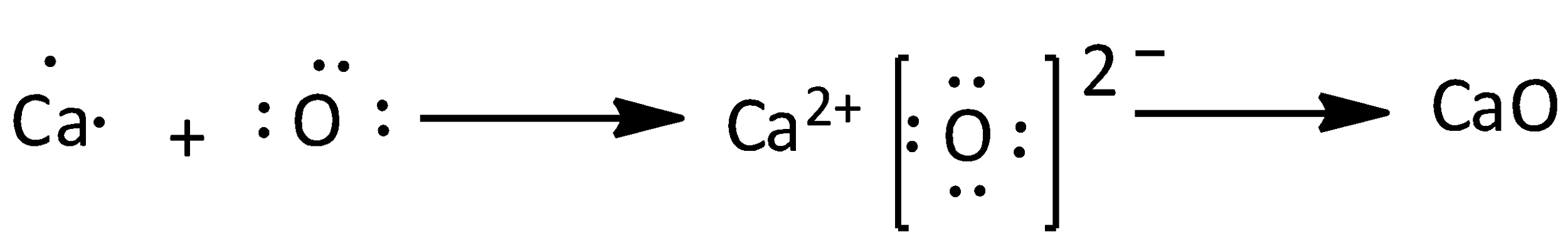
Note: We can use Lewis structure to determine the molecules geometry since it helps us to identify the outermost electrons. We could predict the geometry of a compound by,
Sketching its Lewis structure.
Calculating the number of electron pairs.
Arranging the electron pairs to decrease repulsion.
Arranging the atoms to decrease the repulsion of lone pair-lone pairs.
Naming the molecular geometries from the positions of the atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




