
Write the E – Z configuration of the following compound .
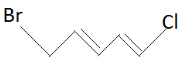

Answer
509.1k+ views
Hint: When the two priority groups are on the same side then the configuration is said to be in Z configuration. While if these two priority groups are on different sides then it is called E configuration. These E and Z configurations are applicable on double bonds only.
Complete answer:
The E-Z configuration is named after analyzing the two groups at each end of a double bond. The groups are lower priority and higher priority groups . The priority of a group is decided by the CIP rules. The first rule is that the atom having higher atomic number will receive the higher priority .This rule is enough to give the answer to our question. Firstly, according to the IUPAC number, it is organic.
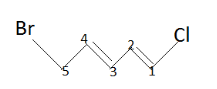
Now according to the CIP rule ,first find a double bond . It is present at $1$ and $3$ positions . Now we have to give priority to groups surrounded by double bonds at these positions.
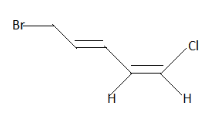
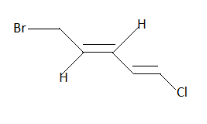
For position $1$ -
At the right side of the double bond we have $Cl$ and $H$ . Since the atomic number of Chlorine $\left( {17} \right)$ is higher than that of hydrogen $\left( 1 \right)$ .Therefore $Cl$ receives higher priority than $H$ . Similarly at the left side we have Carbon atom and Hydrogen. Again due to higher atomic number the carbon atom is at higher priority . Now compare the positions of the groups .We can easily see that higher priority groups at both sides of the double side are at the same side i.e upper side. Hence we can say it is in Z configuration.
Similarly we can give priorities at position $3$ $C$ too. $Br$ and $C$ are higher priorities located on opposite sides. Therefore it is E configuration. Thus it is $1Z{\text{ , 3E}}$ .
Note:
E-Z nomenclature is introduced in place of cis trans . When the cis trans does not work then we have to apply E-Z nomenclature. There are other rules for CIP. We use only one basic rule but there is a complete list of rules for determining the priority of groups.
Complete answer:
The E-Z configuration is named after analyzing the two groups at each end of a double bond. The groups are lower priority and higher priority groups . The priority of a group is decided by the CIP rules. The first rule is that the atom having higher atomic number will receive the higher priority .This rule is enough to give the answer to our question. Firstly, according to the IUPAC number, it is organic.

Now according to the CIP rule ,first find a double bond . It is present at $1$ and $3$ positions . Now we have to give priority to groups surrounded by double bonds at these positions.


For position $1$ -
At the right side of the double bond we have $Cl$ and $H$ . Since the atomic number of Chlorine $\left( {17} \right)$ is higher than that of hydrogen $\left( 1 \right)$ .Therefore $Cl$ receives higher priority than $H$ . Similarly at the left side we have Carbon atom and Hydrogen. Again due to higher atomic number the carbon atom is at higher priority . Now compare the positions of the groups .We can easily see that higher priority groups at both sides of the double side are at the same side i.e upper side. Hence we can say it is in Z configuration.
Similarly we can give priorities at position $3$ $C$ too. $Br$ and $C$ are higher priorities located on opposite sides. Therefore it is E configuration. Thus it is $1Z{\text{ , 3E}}$ .
Note:
E-Z nomenclature is introduced in place of cis trans . When the cis trans does not work then we have to apply E-Z nomenclature. There are other rules for CIP. We use only one basic rule but there is a complete list of rules for determining the priority of groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




