
Write the correct order of acidity:
P.
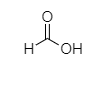
Q.
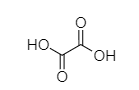
R.
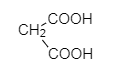
S.
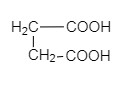
a.) P > Q > R > S
b.) Q > P > R > S
c.) Q > R > S > P
d.) S > R > Q > P


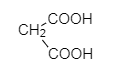
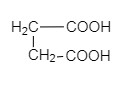
Answer
596.7k+ views
Hint: Acidity is the tendency of a compound to act as ${ H }^{ + }$ donor. The carboxylic acid is acidic as it has hydrogen in the ${ -COOH }$ group. The compound whose carboxylate ion is the more stable will be more acidic.
Complete step by step solution:
Carboxylic acids are acidic in nature due to resonance stabilization of carboxylate ion and hydrogen of ${ -COOH }$, which is found to be capable of ionization. As we know, oxalic acid easily releases its H atom to form ${ CH_{ 3 }COO }^{ - }$ ion whereas formic acid needs more energy. Hence, the Strength of oxalic acid is more than formic acid.
So, Oxalic acid (COOH-COOH) is more acidic than formic acid (${ HCOOH }$). Malonic acid, (${ COOH-CH }_{ 2 }{ -COOH }$) is less acidic than oxalic acid because of the intervening presence of ${ -CH }_{ 2 }$ group and succinic acid (${ COOH-CH }_{ 2 }{ { -CH }_{ 2 }-COOH }$) which is much weaker than (${ COOH-CH }_{ 2 }{ -COOH }$). Malonic acid due to the greater distance created by ${ -CH }_{ 2 }$ group.
Hence, the order of acidity will be:
COOH-COOH > HCOOH > COOH-CH$_2$-COOH > COOH-CH$_2$-CH$_2$-COOH
So, the correct order is QP R S and hence, the correct option is B.
Additional Information:
Carboxylic acids are important in the manufacture of greases, plastics, and crayons.
Carboxylic acids exhibit strong hydrogen bonding between the molecules.
Note: The possibility to make a mistake is that you can choose option D. ${ COOH-COOH }$ is more acidic than ${ COOH-CH }_{ 2 }{ -COOH }$ due to +I effect which causes a decrease in acidity.
Complete step by step solution:
Carboxylic acids are acidic in nature due to resonance stabilization of carboxylate ion and hydrogen of ${ -COOH }$, which is found to be capable of ionization. As we know, oxalic acid easily releases its H atom to form ${ CH_{ 3 }COO }^{ - }$ ion whereas formic acid needs more energy. Hence, the Strength of oxalic acid is more than formic acid.
So, Oxalic acid (COOH-COOH) is more acidic than formic acid (${ HCOOH }$). Malonic acid, (${ COOH-CH }_{ 2 }{ -COOH }$) is less acidic than oxalic acid because of the intervening presence of ${ -CH }_{ 2 }$ group and succinic acid (${ COOH-CH }_{ 2 }{ { -CH }_{ 2 }-COOH }$) which is much weaker than (${ COOH-CH }_{ 2 }{ -COOH }$). Malonic acid due to the greater distance created by ${ -CH }_{ 2 }$ group.
Hence, the order of acidity will be:
COOH-COOH > HCOOH > COOH-CH$_2$-COOH > COOH-CH$_2$-CH$_2$-COOH
So, the correct order is QP R S and hence, the correct option is B.
Additional Information:
Carboxylic acids are important in the manufacture of greases, plastics, and crayons.
Carboxylic acids exhibit strong hydrogen bonding between the molecules.
Note: The possibility to make a mistake is that you can choose option D. ${ COOH-COOH }$ is more acidic than ${ COOH-CH }_{ 2 }{ -COOH }$ due to +I effect which causes a decrease in acidity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




