
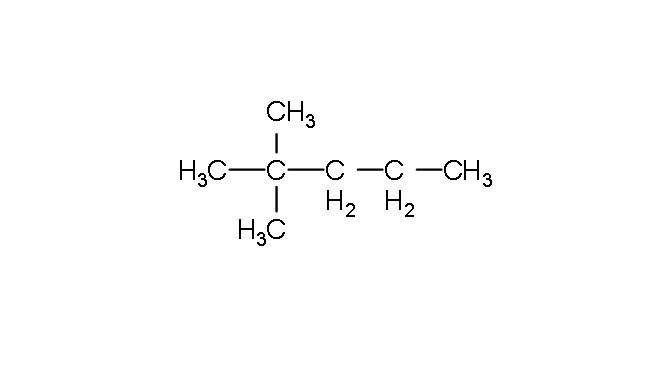
Write the common name of the given compound.

Answer
566.7k+ views
Hint: In order to find the common name of the given carbon compound, first we will try to determine its IUPAC nomenclature and the process to give an IUPAC name of any carbon structure. Then according to the IUPAC rules, we can find the name of the given structure.
Complete Solution :
To write the IUPAC name of any compound ,we can follow the following sets of rules
(i) The parent chain or longest carbon chain should be identified.
(ii) The groups appending from the parent chain(substituents) should be identified.
(iii) The carbon atoms of the parent chain should be numbered from the end which gives the substituents the lowest number.
(iv) If the substituent occurs more than once, then the position is given for any point where the substituent occurs and further a suffix (di,tri,tetra etc) specifies the number of times the substituent appears in the carbon chain.
- Now let's look at the given compound. From the structure we can see that the number of carbon in the longest chain is 5 and so the name will be pentane. As we can see there are two methyl ($C{{H}_{3}}$) groups attached to the second carbon atom and hence the name will be 2,2-Dimethylpentane.
Therefore the IUPAC name of the given compound is 2,2-Dimethylpentane and since it contains ${{\left( C{{H}_{3}} \right)}_{3}}C$ grouping its also called as neoheptane .Therefore the common name of the given compound is neoheptane.
Note: Keep in mind that the compound 2,2-Dimethylpentane is one of the isomers of heptane and it is also called as neoheptane since it contains the ${{\left( C{{H}_{3}} \right)}_{3}}C$ grouping. Also,it should be noted that it has the most extreme properties of the isomers.
Complete Solution :
To write the IUPAC name of any compound ,we can follow the following sets of rules
(i) The parent chain or longest carbon chain should be identified.
(ii) The groups appending from the parent chain(substituents) should be identified.
(iii) The carbon atoms of the parent chain should be numbered from the end which gives the substituents the lowest number.
(iv) If the substituent occurs more than once, then the position is given for any point where the substituent occurs and further a suffix (di,tri,tetra etc) specifies the number of times the substituent appears in the carbon chain.
- Now let's look at the given compound. From the structure we can see that the number of carbon in the longest chain is 5 and so the name will be pentane. As we can see there are two methyl ($C{{H}_{3}}$) groups attached to the second carbon atom and hence the name will be 2,2-Dimethylpentane.
Therefore the IUPAC name of the given compound is 2,2-Dimethylpentane and since it contains ${{\left( C{{H}_{3}} \right)}_{3}}C$ grouping its also called as neoheptane .Therefore the common name of the given compound is neoheptane.
Note: Keep in mind that the compound 2,2-Dimethylpentane is one of the isomers of heptane and it is also called as neoheptane since it contains the ${{\left( C{{H}_{3}} \right)}_{3}}C$ grouping. Also,it should be noted that it has the most extreme properties of the isomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




