
Write the chemical equation for preparing sodium soap from glyceryl oleate and glyceryl palmitate. Structural formulas for the given compounds are given below.
i)\[({{C}_{15}}{{H}_{31}}COO){{C}_{3}}{{H}_{5}}\] - Glyceryl palmitate
ii)${{({{C}_{17}}{{H}_{32}}COO)}_{3}}{{C}_{3}}{{H}_{5}}$- Glyceryl oleate
Answer
570k+ views
Hint: In the question the reactions are required for preparation of sodium soap from glycerol oleate and glyceryl palmitate. The type of alkali metal used to make the soap determines the kind of soap. Sodium soap is made from sodium hydroxide.
Complete step by step solution:
In this question we will write the required reaction:
In the first we will see the reaction for the formation of sodium soap from glyceryl palmitate.
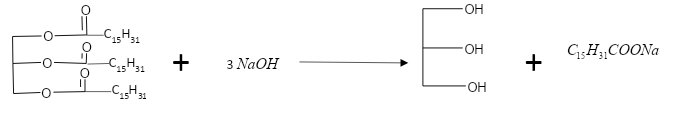
This is the required reaction.
In the next part of the question we are asked about the reaction to make sodium soap from glyceryl oleate.

This is the required reaction.In both the reactions Glycerol is formed along with the required sodium soap.
Additional information:
In industrial settings, soaps are used as thickeners, components of some lubricants, and precursors to catalysts. When used for cleaning, soap solubilizes particles and grime, which can then be separated from the article being cleaned. In hand washing, as a surfactant, when lathered with a little water, soap kills microorganisms by ripping open the cellular membrane lipid bilayer and denaturing their proteins. It also emulsifies oils, enabling them to be carried away by running water. Soap is made by mixing fats and oils with a base, as opposed to detergent which is created by combining chemical compounds in a mixer.
Note: In this question we were required to provide only the reaction. So for solving this type of question the reactions should be known. The structures should be known. Also it should be noted that the glycerol will be formed in this kind of reaction for forming soap.
Complete step by step solution:
In this question we will write the required reaction:
In the first we will see the reaction for the formation of sodium soap from glyceryl palmitate.

This is the required reaction.
In the next part of the question we are asked about the reaction to make sodium soap from glyceryl oleate.

This is the required reaction.In both the reactions Glycerol is formed along with the required sodium soap.
Additional information:
In industrial settings, soaps are used as thickeners, components of some lubricants, and precursors to catalysts. When used for cleaning, soap solubilizes particles and grime, which can then be separated from the article being cleaned. In hand washing, as a surfactant, when lathered with a little water, soap kills microorganisms by ripping open the cellular membrane lipid bilayer and denaturing their proteins. It also emulsifies oils, enabling them to be carried away by running water. Soap is made by mixing fats and oils with a base, as opposed to detergent which is created by combining chemical compounds in a mixer.
Note: In this question we were required to provide only the reaction. So for solving this type of question the reactions should be known. The structures should be known. Also it should be noted that the glycerol will be formed in this kind of reaction for forming soap.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




