
Write the balanced equation for the chemical reaction in which Phosphorus burns in Chlorine gas to give out Phosphorus Pentachloride.
Answer
601.2k+ views
Hint: Remember that Phosphorus exists in its natural state as a ${{P}_{4}}$ molecule. Also remember that the oxidation state of Phosphorus in Phosphorus Pentachloride is +5.
Step-by-Step Solution:
Let us study the chemical and physical properties of Phosphorus Pentachloride before studying its origination reaction i.e. the one concerning this question.
Phosphorus pentachloride is the chemical compound with the formula \[PC{{l}_{5}}\]. It is one of the most important phosphorus chlorides, with the others being \[PC{{l}_{3}}\] and \[POC{{l}_{3}}\], and it is primarily used as a chlorinating reagent. It is a colourless, water-sensitive and moisture-sensitive solid, although commercial samples can be yellowish and contaminated with HCl.
The structure of \[PC{{l}_{5}}\] is very much along the lines of the VSEPR theory and is observed to be as follows:
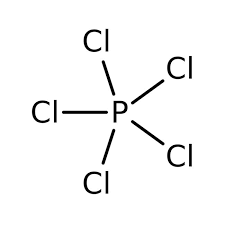
Now, while the primary method of the preparation of \[PC{{l}_{5}}\] involves the chlorination of \[PC{{l}_{3}}\]; it can also be prepared by the burning of Phosphorus in the presence of Chlorine gas, the reaction for which can be written as follows:
${{P}_{4}}+10C{{l}_{2}}\xrightarrow{\Delta }4PC{{l}_{5}}$
Which also happens to be the required chemical equation to complete this solution.
Note: In its most characteristic reaction, \[PC{{l}_{5}}\] reacts upon contact with water to release hydrogen chloride and give phosphorus oxides. The first hydrolysis product is phosphorus oxychloride (\[POC{{l}_{3}}\]) whereas in hot water, it reacts to produce orthophosphoric acid (${{H}_{3}}P{{O}_{4}}$).
Step-by-Step Solution:
Let us study the chemical and physical properties of Phosphorus Pentachloride before studying its origination reaction i.e. the one concerning this question.
Phosphorus pentachloride is the chemical compound with the formula \[PC{{l}_{5}}\]. It is one of the most important phosphorus chlorides, with the others being \[PC{{l}_{3}}\] and \[POC{{l}_{3}}\], and it is primarily used as a chlorinating reagent. It is a colourless, water-sensitive and moisture-sensitive solid, although commercial samples can be yellowish and contaminated with HCl.
The structure of \[PC{{l}_{5}}\] is very much along the lines of the VSEPR theory and is observed to be as follows:

Now, while the primary method of the preparation of \[PC{{l}_{5}}\] involves the chlorination of \[PC{{l}_{3}}\]; it can also be prepared by the burning of Phosphorus in the presence of Chlorine gas, the reaction for which can be written as follows:
${{P}_{4}}+10C{{l}_{2}}\xrightarrow{\Delta }4PC{{l}_{5}}$
Which also happens to be the required chemical equation to complete this solution.
Note: In its most characteristic reaction, \[PC{{l}_{5}}\] reacts upon contact with water to release hydrogen chloride and give phosphorus oxides. The first hydrolysis product is phosphorus oxychloride (\[POC{{l}_{3}}\]) whereas in hot water, it reacts to produce orthophosphoric acid (${{H}_{3}}P{{O}_{4}}$).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




