
Write the atomic weight of chlorine isotopes.
Answer
600.3k+ views
Hint: To write the atomic weights of isotopes, we should first know about the definition and examples of isotopes. We should also know about chlorine.
Step by step answer:
Let us first know about isotopes. We should know that isotopes are various forms of an element that have the same atomic number but different atomic mass. We know that the atomic mass of an element is equal to the number of electrons present in its atom. Or we can say that the atomic number of an element is equal to the number of protons present in its atom. And as we know that in case of atomic mass, the sum of the number of protons and number of neutrons that are present in the nuclei of an atom are equal to the atomic mass.
From the above paragraph we can understand the isotope with a new direction. We can say that isotopes are any of two or more forms of an element where the atoms have the same number of protons, but a different number of neutrons within their nuclei. Or we can say that atomic mass is different and atomic mass is equal in isotopes.
Let us understand this by one example of carbon. This is the example of hydrogen and their isotopes.
In the same way we will find isotopes in the case of chlorine.
In the above table we had successfully mentioned the isotopes of chlorine.
Note: We should note that we are using chlorine stable isotopes as a tool for tracing the origin and fate of fluids and rocks from the Earth's surface and interior. For example we can find the fluid movement in sedimentary basins.
We should know that isotopes undergo spontaneous decay during which they emit radiation and achieve a stable state. This property of radioisotopes is useful in food preservation, archaeological dating of artefacts and medical diagnosis and treatment.
Step by step answer:
Let us first know about isotopes. We should know that isotopes are various forms of an element that have the same atomic number but different atomic mass. We know that the atomic mass of an element is equal to the number of electrons present in its atom. Or we can say that the atomic number of an element is equal to the number of protons present in its atom. And as we know that in case of atomic mass, the sum of the number of protons and number of neutrons that are present in the nuclei of an atom are equal to the atomic mass.
From the above paragraph we can understand the isotope with a new direction. We can say that isotopes are any of two or more forms of an element where the atoms have the same number of protons, but a different number of neutrons within their nuclei. Or we can say that atomic mass is different and atomic mass is equal in isotopes.
Let us understand this by one example of carbon. This is the example of hydrogen and their isotopes.
| Isotope | Atomic number | Atomic mass | Proton | Electron | Neutron | representation |
| hydrogen | 1 | 1 | 1 | 1 | 0 | 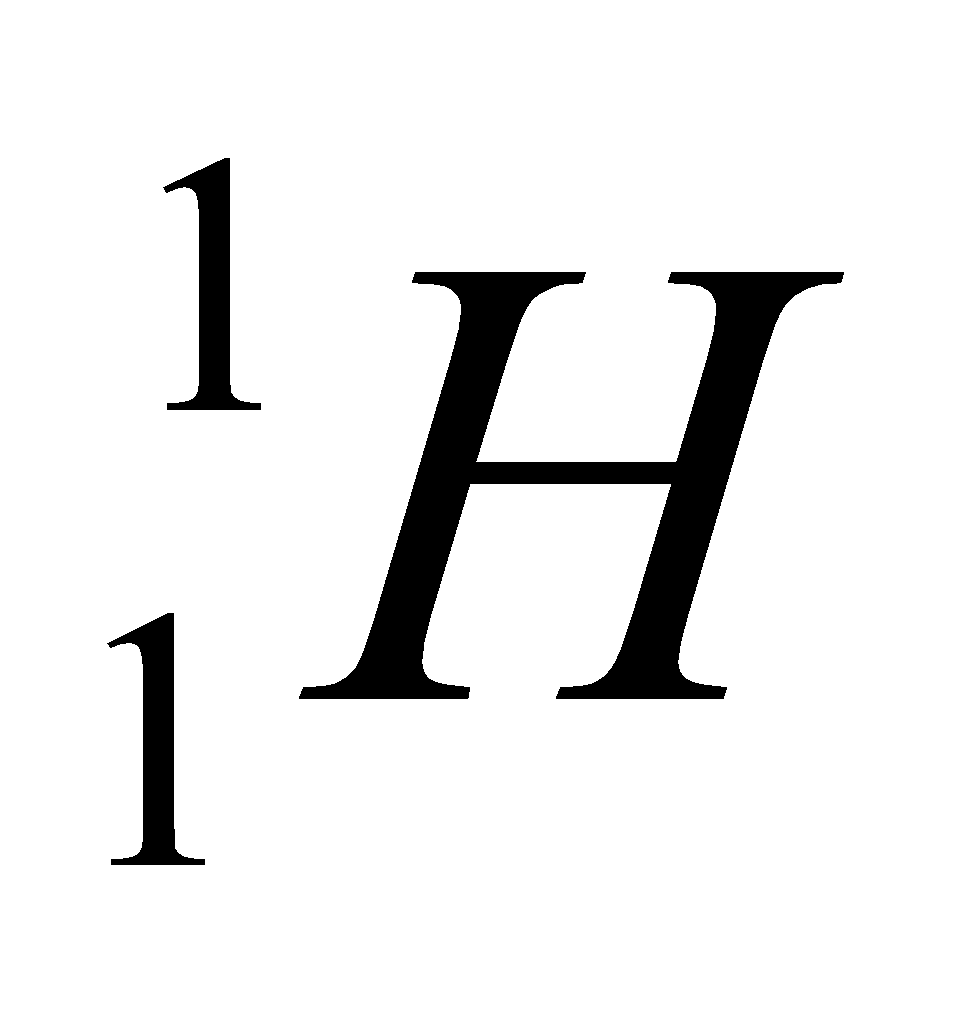
|
| deuterium | 1 | 2 | 1 | 1 | 1 | 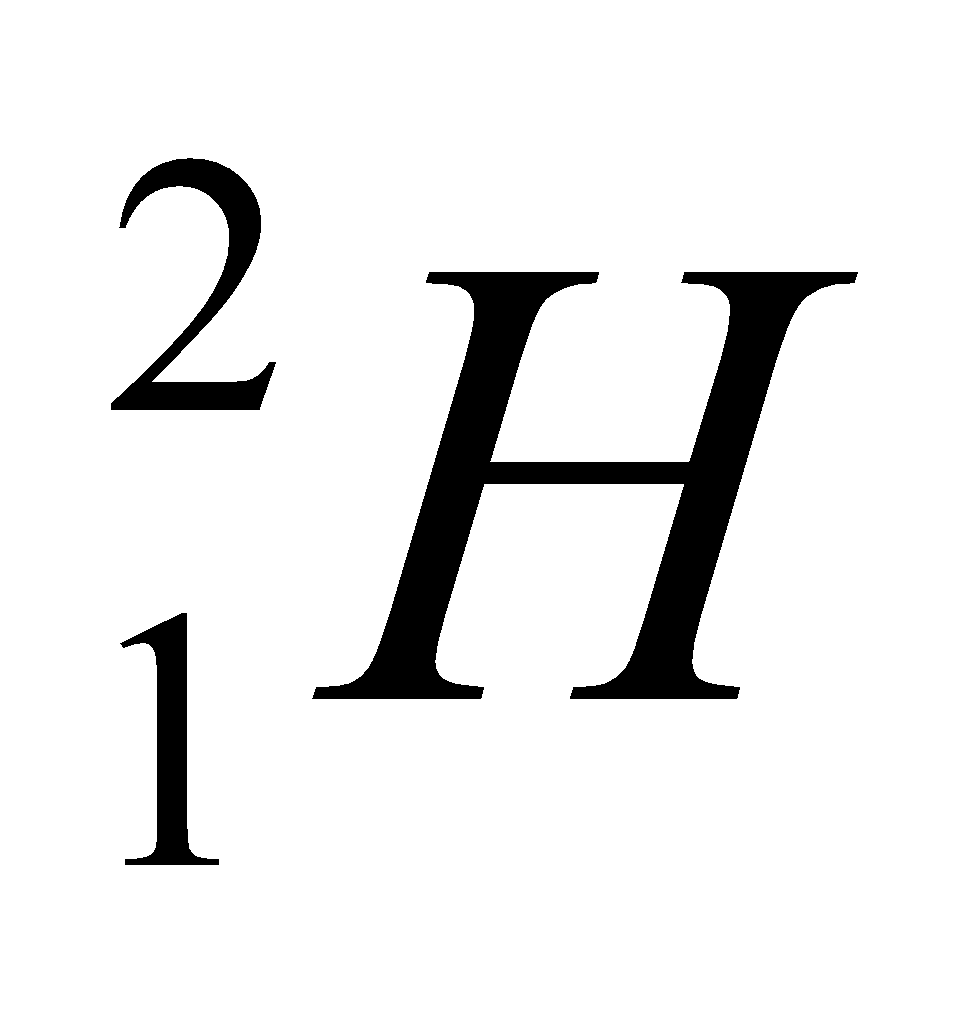
|
| tritium | 1 | 3 | 1 | 1 | 2 | 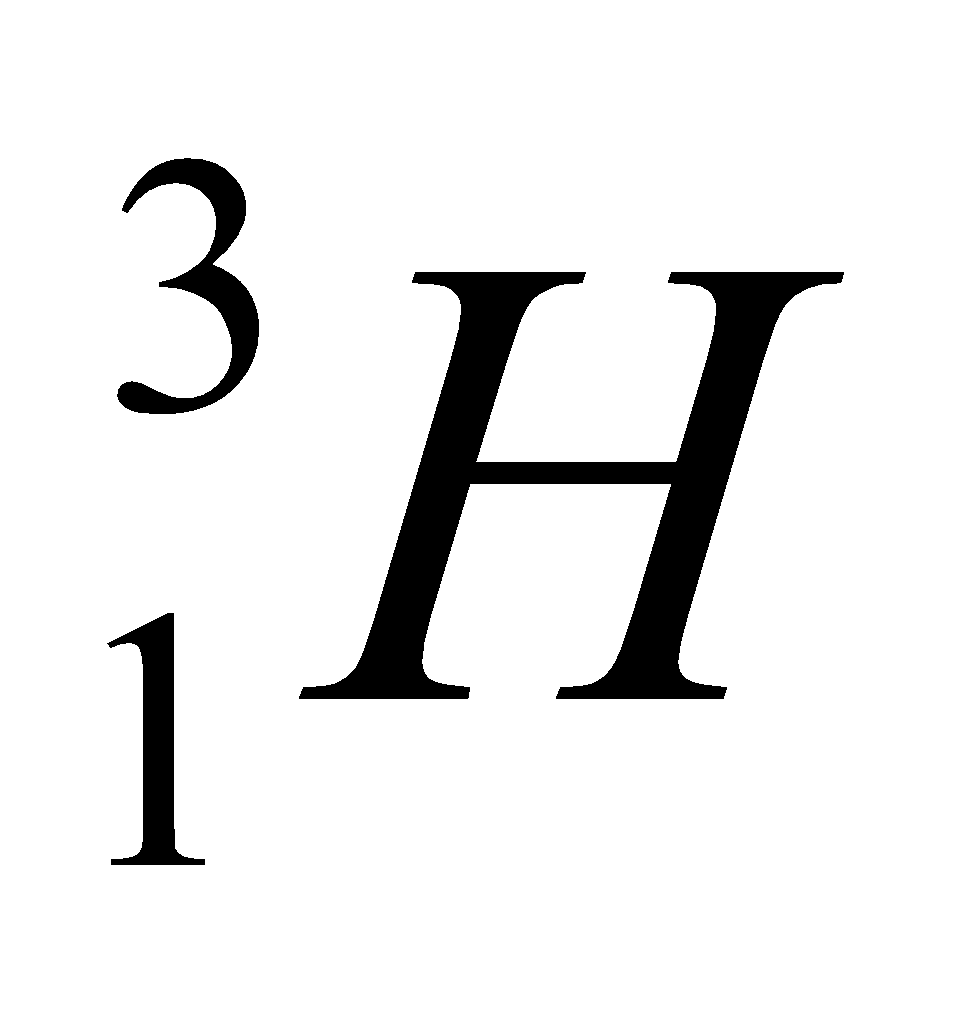
|
In the same way we will find isotopes in the case of chlorine.
| Isotope | Atomic number | Atomic mass | Proton | Electron | Neutron | representation |
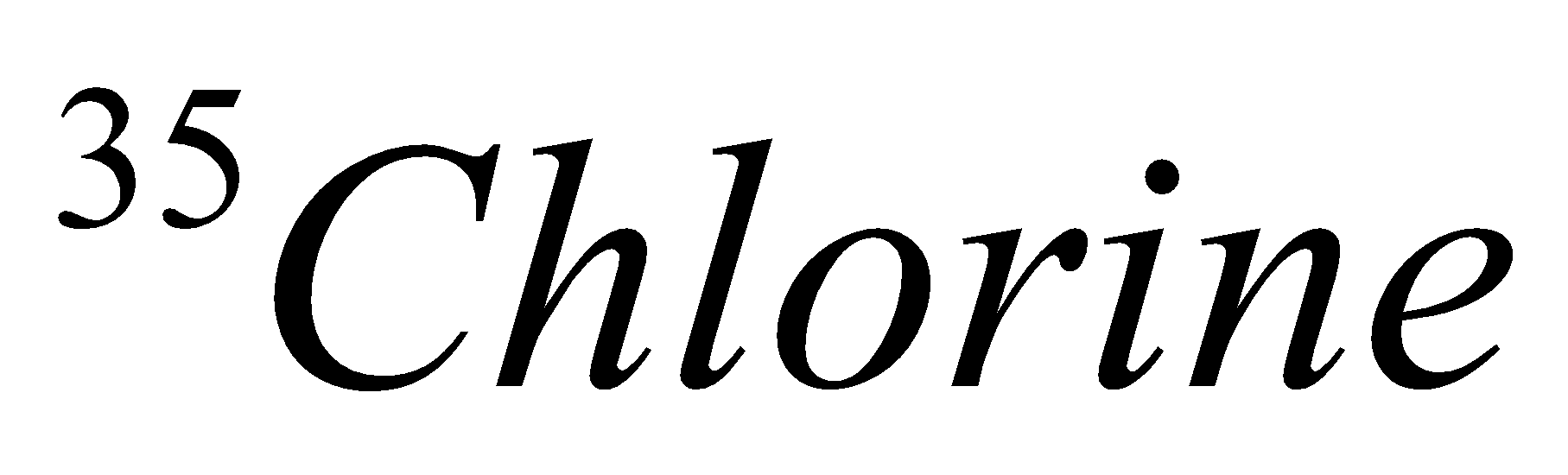
| 17 | 35 | 17 | 17 | 18 | 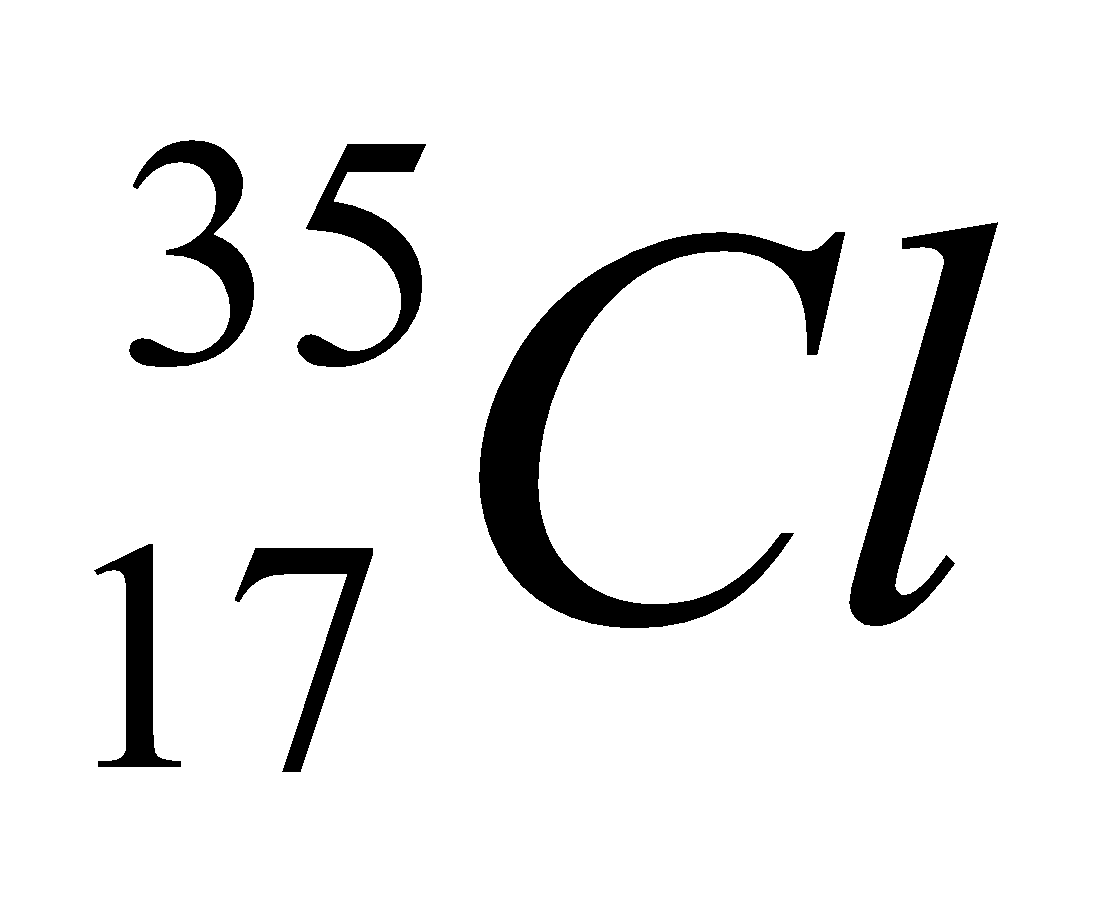
|
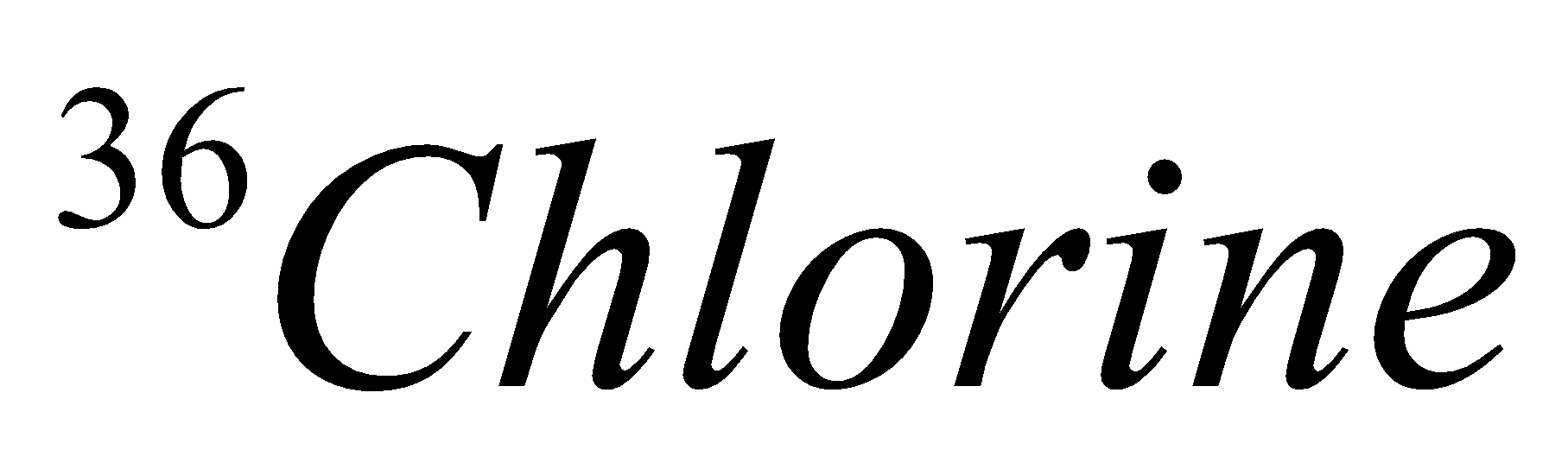

| 17 | 36 | 17 | 17 | 19 | 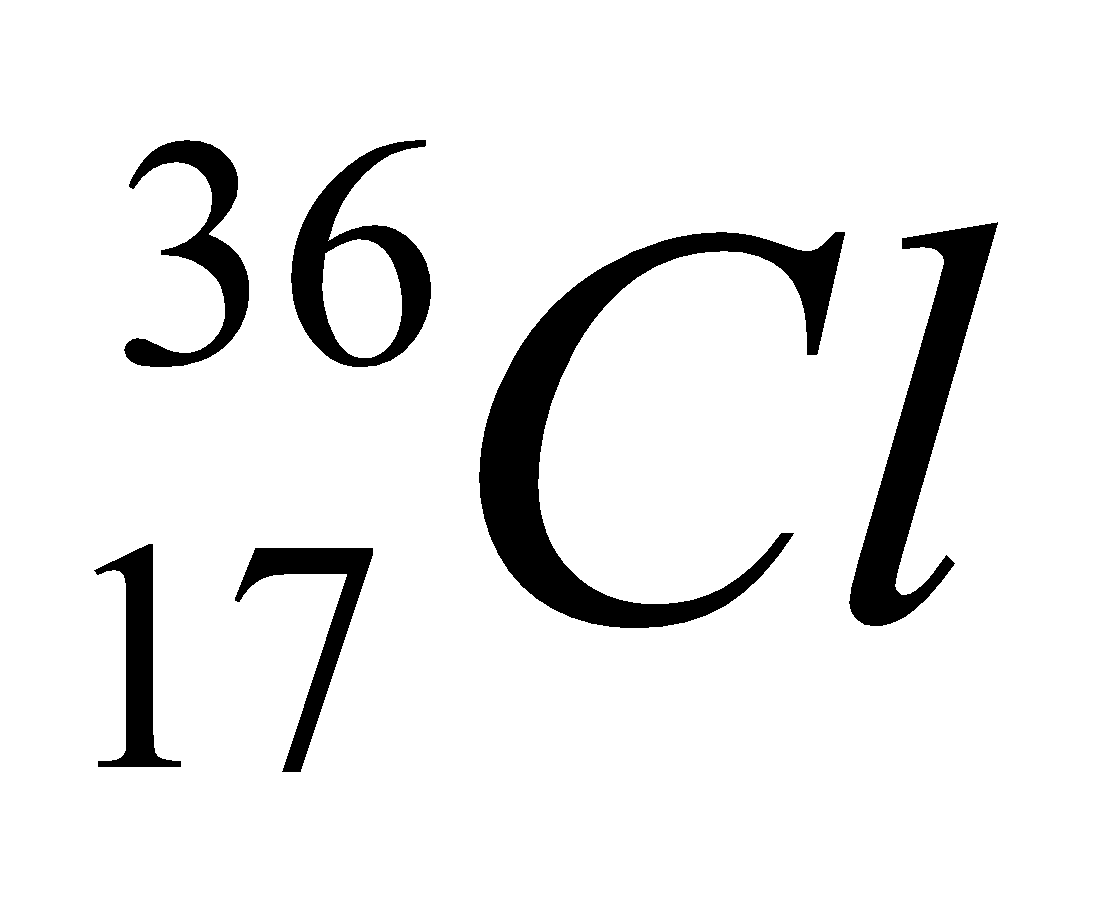
|
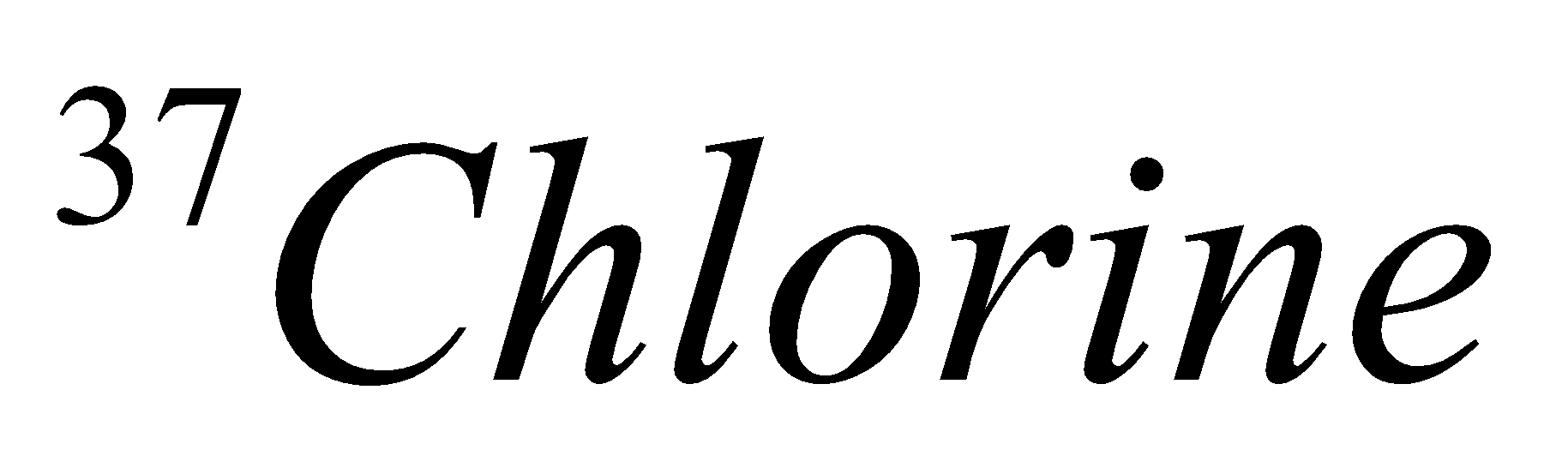
| 17 | 337 | 17 | 17 | 20 | 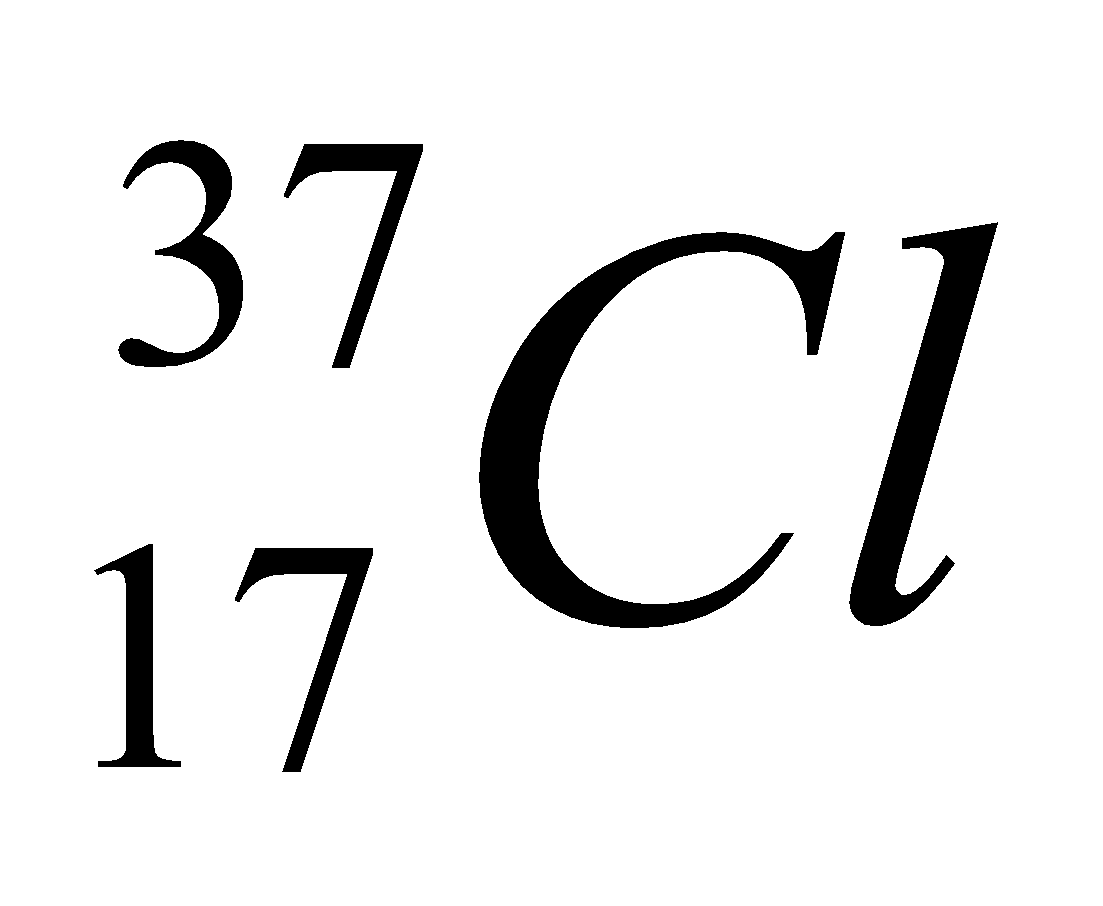
|
In the above table we had successfully mentioned the isotopes of chlorine.
Note: We should note that we are using chlorine stable isotopes as a tool for tracing the origin and fate of fluids and rocks from the Earth's surface and interior. For example we can find the fluid movement in sedimentary basins.
We should know that isotopes undergo spontaneous decay during which they emit radiation and achieve a stable state. This property of radioisotopes is useful in food preservation, archaeological dating of artefacts and medical diagnosis and treatment.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




