
Write steps for reduction of ester to alcohol.
Answer
530.7k+ views
Hint: Reduction means there will be either removal of oxygen atoms or addition of hydrogen atom. Esters are the organic compound in which the functional group present is –COO- between two alkyl groups. Alcohols are the organic compound in which the functional group is –OH, and the hydroxyl group can terminal as well as the center of the carbon chain. The reduction will take place in two steps.
Complete answer:
There are many organic compounds that can be distinguished by the presence of functional groups present in them, like alkanes, carboxylic acids, alcohols, esters, ketones, aldehydes, etc. The functional groups can be converted into each other by oxidation and reduction process.
Reduction means there will be either removal of oxygen atoms or the addition of hydrogen atoms. Esters are the organic compound in which the functional group present is –COO- between two alkyl groups. Alcohols are the organic compound in which the functional group is –OH, and the hydroxyl group can terminal as well as the center of the carbon chain.
Reduction is done by compounds like lithium aluminum hydride, etc. So, when the ester reacts with lithium aluminium hydride, then first it will be converted into aldehyde and then this aldehyde is further reduced by lithium aluminium hydride to be converted into alcohol. The reaction is given below:
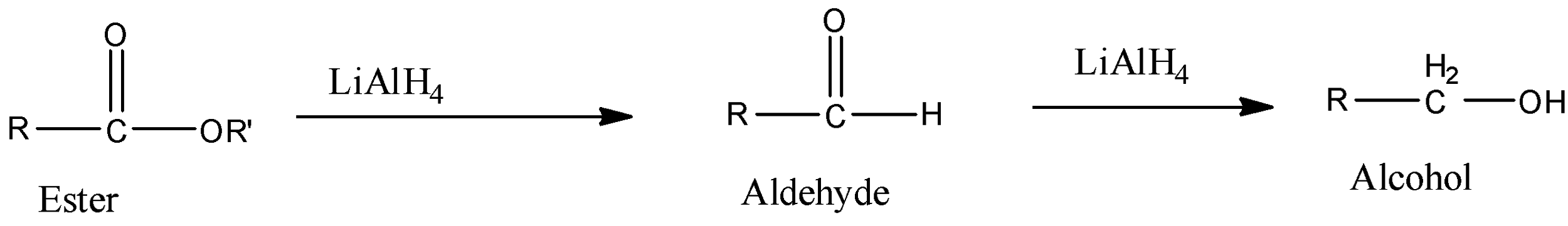
Therefore, the esters are converted into alcohol in two steps.
Note:
When the compound is oxidized then there is the removal of hydrogen atoms or addition of oxygen atoms, if the esters are oxidized then it will be converted into a carboxylic acid.
Complete answer:
There are many organic compounds that can be distinguished by the presence of functional groups present in them, like alkanes, carboxylic acids, alcohols, esters, ketones, aldehydes, etc. The functional groups can be converted into each other by oxidation and reduction process.
Reduction means there will be either removal of oxygen atoms or the addition of hydrogen atoms. Esters are the organic compound in which the functional group present is –COO- between two alkyl groups. Alcohols are the organic compound in which the functional group is –OH, and the hydroxyl group can terminal as well as the center of the carbon chain.
Reduction is done by compounds like lithium aluminum hydride, etc. So, when the ester reacts with lithium aluminium hydride, then first it will be converted into aldehyde and then this aldehyde is further reduced by lithium aluminium hydride to be converted into alcohol. The reaction is given below:

Therefore, the esters are converted into alcohol in two steps.
Note:
When the compound is oxidized then there is the removal of hydrogen atoms or addition of oxygen atoms, if the esters are oxidized then it will be converted into a carboxylic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




