
Write short notes on the following:
-Claisen condensation
-Benzoin condensation
Answer
594.9k+ views
Hint: Claisen condensation involves self condensation of esters in presence of base. Two compounds having aldehyde functional groups can form benzoin which is called benzoin condensation.
Complete Answer:
-> It is known to you that claisen condensation is a self condensation and benzoin condensation is a reaction between two aldehydes. Let’s see both of the reactions one by one.
-> Claisen condensation:
It involves self-condensation of two molecules of ester containing alpha-hydrogen in the presence of a strong base such as sodium ethoxide to form beta-keto ester. The esters undergo self condensation to produce beta-keto esters. Mixed or crossed claisen condensation also occurs between two different esters or between an ester and a ketone. The reaction occurs in four steps to produce the desired product. Below is an example of this reaction. We can see that a $\beta -$ keto ester is formed as an end product.
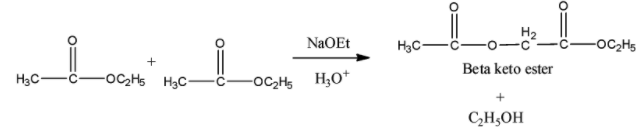
- Benzoin condensation:
Two molecules of aromatic aldehyde (such as benzaldehyde), on heating in the presence of ethanolic KCN get condensed to form benzoin. The catalyst cyanide ion acts as a good nucleophilic attacker which can promote nucleophilicity of the intermediate and also act as a good leaving group. Benzoin condensation is a difficult reaction, for the reason that there is possibility of benzaldehyde to be oxidized into benzoic acid. Below is the reaction of two benzaldehyde molecules reacting to give Benzoin
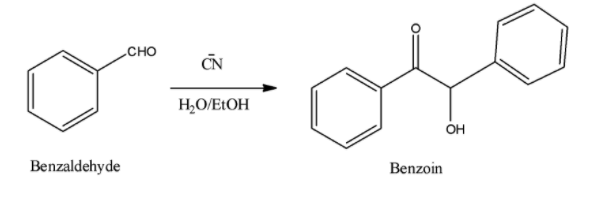
Note: Remember that $\alpha $ hydrogen is a necessary requirement for the ester to undergo claisen condensation reaction. Remember that aliphatic aldehydes cannot undergo benzoin condensation as the aldehyde needs to be aromatic in order to give benzoin as a final product.
Complete Answer:
-> It is known to you that claisen condensation is a self condensation and benzoin condensation is a reaction between two aldehydes. Let’s see both of the reactions one by one.
-> Claisen condensation:
It involves self-condensation of two molecules of ester containing alpha-hydrogen in the presence of a strong base such as sodium ethoxide to form beta-keto ester. The esters undergo self condensation to produce beta-keto esters. Mixed or crossed claisen condensation also occurs between two different esters or between an ester and a ketone. The reaction occurs in four steps to produce the desired product. Below is an example of this reaction. We can see that a $\beta -$ keto ester is formed as an end product.

- Benzoin condensation:
Two molecules of aromatic aldehyde (such as benzaldehyde), on heating in the presence of ethanolic KCN get condensed to form benzoin. The catalyst cyanide ion acts as a good nucleophilic attacker which can promote nucleophilicity of the intermediate and also act as a good leaving group. Benzoin condensation is a difficult reaction, for the reason that there is possibility of benzaldehyde to be oxidized into benzoic acid. Below is the reaction of two benzaldehyde molecules reacting to give Benzoin

Note: Remember that $\alpha $ hydrogen is a necessary requirement for the ester to undergo claisen condensation reaction. Remember that aliphatic aldehydes cannot undergo benzoin condensation as the aldehyde needs to be aromatic in order to give benzoin as a final product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




